Gastrointestinal Transit Model
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Drug-related gastrointestinal side effects can cause a great deal of discomfort and pain. These side effects include constipation, GI irritation, abdominal constriction or diarrhea.
Similar to the Colonic Propulsion Motility model, the Gastrointestinal Transit Model (sometimes call the Charcoal Meal Model) is also commonly used to evaluate bowel motility and further reflect GI function.
At Melior, we have validated this model by examining morphine’s effect on inhibiting gastrointestinal motility.
Ready to get started or looking for a custom model?
Contact us today for more information about our bespoke research models and to discuss how we can help you answer your unique research questions.
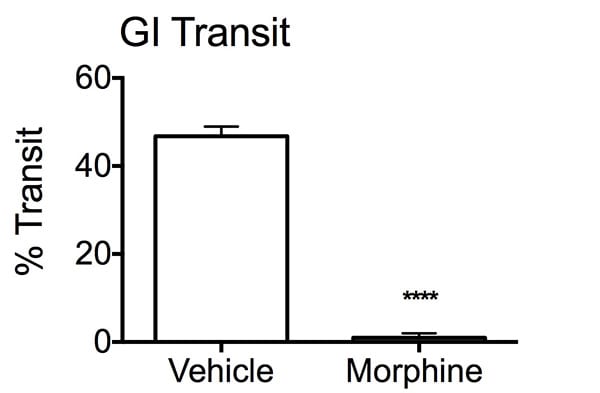
Percent transit of charcoal bolus in the intestines. After an overnight fast, mice were treated with either vehicle or Morphine, 30 minutes prior to charcoal administration. Twenty minutes later, stomach and intestines were removed from the mice and uncoiled to determine the distance travelled by the charcoal bolus through the GI tract. The Morphine group displayed a reduction in percent transit of the bolus compared to the vehicle treated mice. Data are mean ± SEM; ****p<0.001 compared to vehicle (N=10).
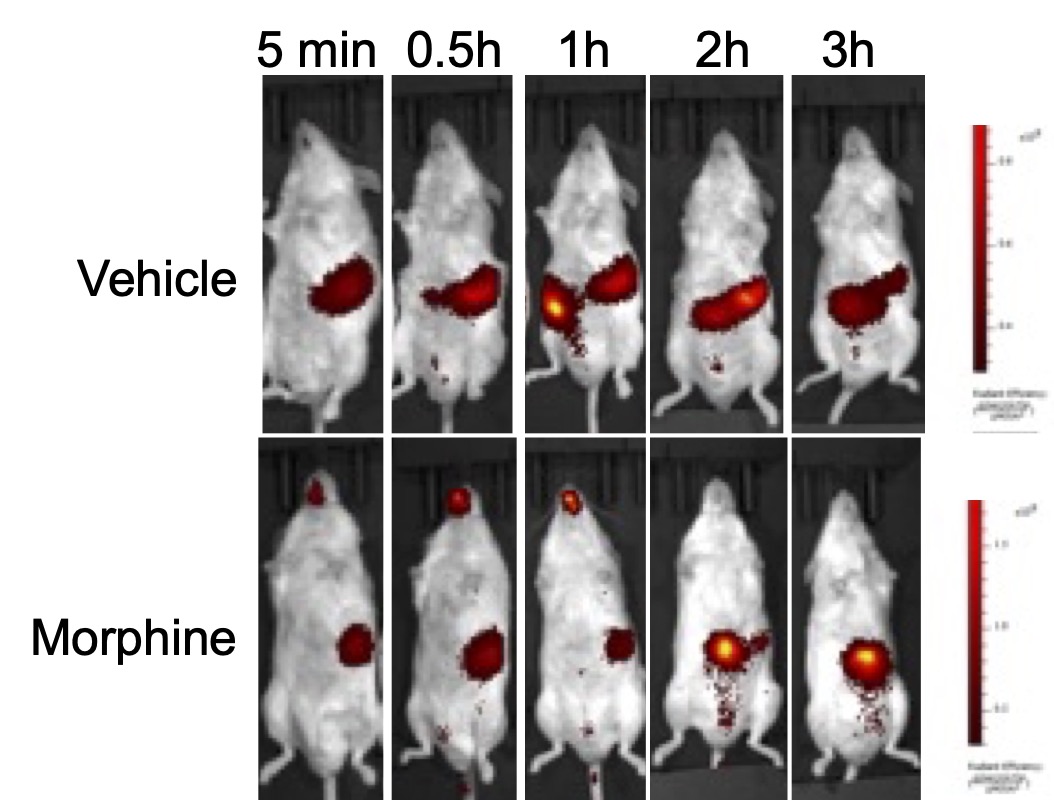
Live Imagining of gastric emptying with GastroSense750. After an overnight fast, mice were treated with either vehicle or Morphine, 30 minutes prior to GastroSense750 administration by oral gavage. A time course of fluorescence imaging was done by IVIS lumina series iii. The Morphine-treated mouse displayed a retention of GastroSense750 compared to the vehicle-treated mouse.
Like other GI motility models the Gastrointestinal Transit model, is typically a one-day model evaluating acute effects of test articles (a single administration). It has relatively low variability typically yielding statistically significant effects with group sizes of 6 to 8 animals. It works well in both rats and mice.



 Interested in running a Gastrointestinal Transit study?
Interested in running a Gastrointestinal Transit study?