Gastrointestinal Transit Model
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Drug-related gastrointestinal side effects can cause a great deal of discomfort and pain. These side effects include constipation, GI irritation, abdominal constriction or diarrhea.
Similar to the Colonic Propulsion Motility model, the Gastrointestinal Transit Model (sometimes call the Charcoal Meal Model) is also commonly used to evaluate bowel motility and further reflect GI function.
At Melior, we have validated this model by examining an opioid’s effect on inhibiting gastrointestinal motility.
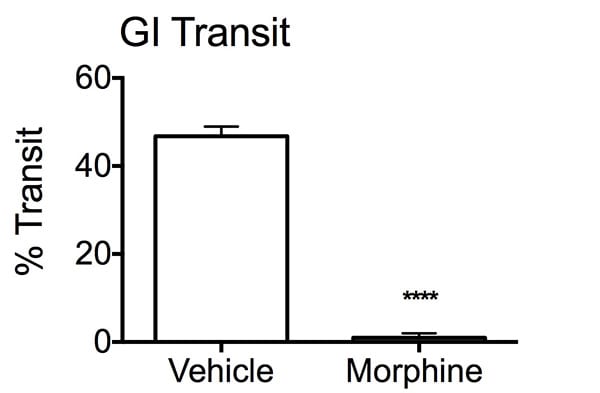
Percent transit of charcoal bolus in the intestines. After an overnight fast, mice were treated with either vehicle or MORPH, 30 minutes prior to charcoal administration. Twenty minutes later, stomach and intestines were removed from the mice and uncoiled to determine the distance travelled by the charcoal bolus through the GI tract. The MORPH group displayed a reduction in percent transit of the bolus compared to the vehicle treated mice. Data are mean ± SEM; ****p<0.001 compared to vehicle (N=10).
Like other GI motility models the Gastrointestinal Transit model, is typically a one-day model evaluating acute effects of test articles (a single administration). It has relatively low variability typically yielding statistically significant effects with group sizes of 6 to 8 animals. It works well in both rats and mice.



 Interested in running a Gastrointestinal Transit study?
Interested in running a Gastrointestinal Transit study?