LPS Model of Sepsis
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Bolus injection of lipopolysaccharide (LPS; endotoxin) a major component of the bacterial cell wall, results in the rapid and transient rise in tumor necrosis factor (TNFα) levels in serum in mammals.
The LPS model of sepsis was originally developed to mirror certain aspects of septic shock in humans. Although this model recapitulates some aspects of sepsis, the correlation between agents effective in rodent LPS models and the clinical septic shock is poor. Nonetheless, this model may be an effective first-line general inflammation model and may be used to determine the anti-inflammatory potential of test compounds.
A variety of clinically approved anti-inflammatory compounds are extremely effective in LPS inflammation including glucocorticoids, NSAIDS and cox-2 inhibitors. We used methylprednisolone (dexamethasone) treatment to pharmacologically validate this model of LPS-mediated TNFα induction.
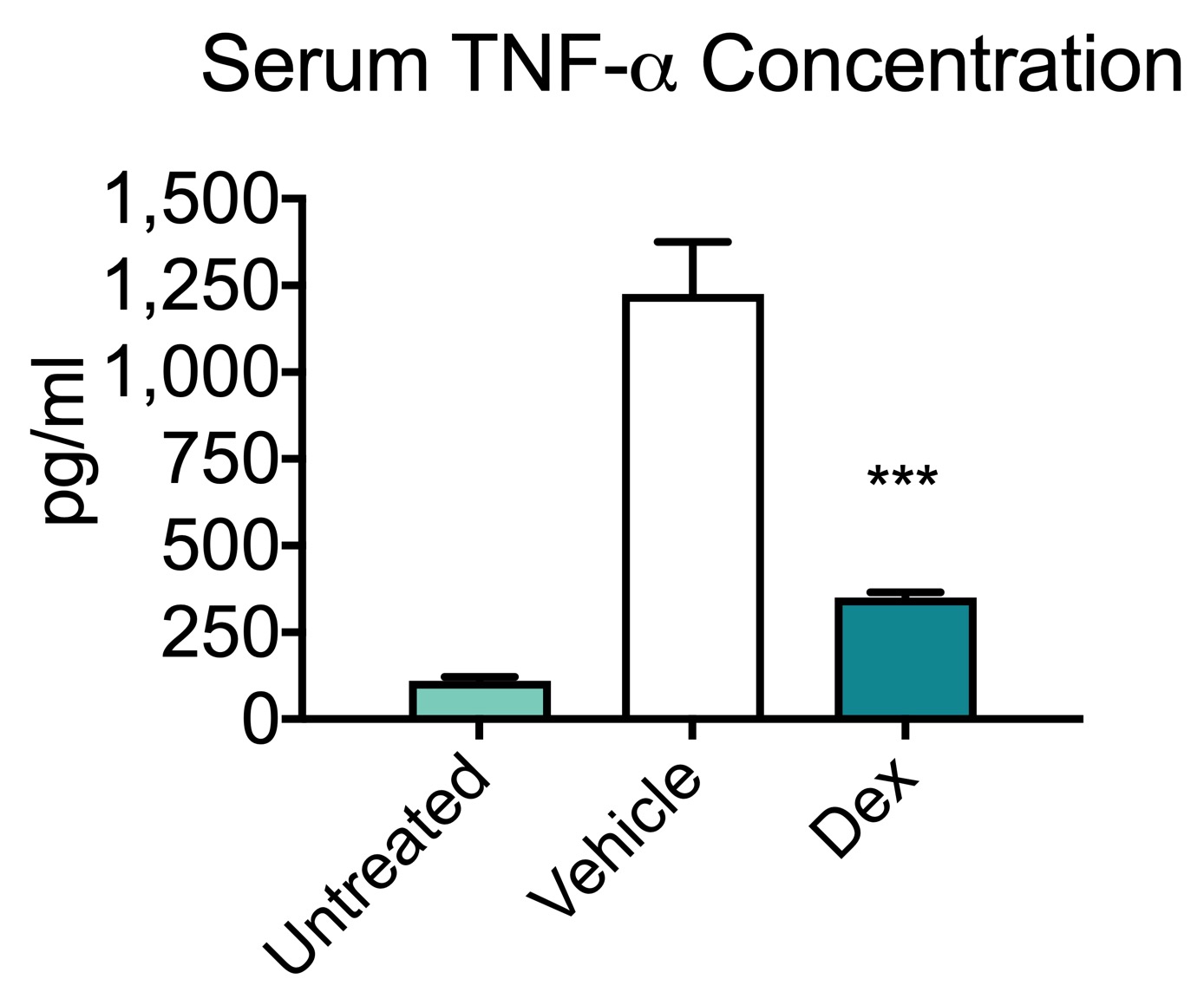
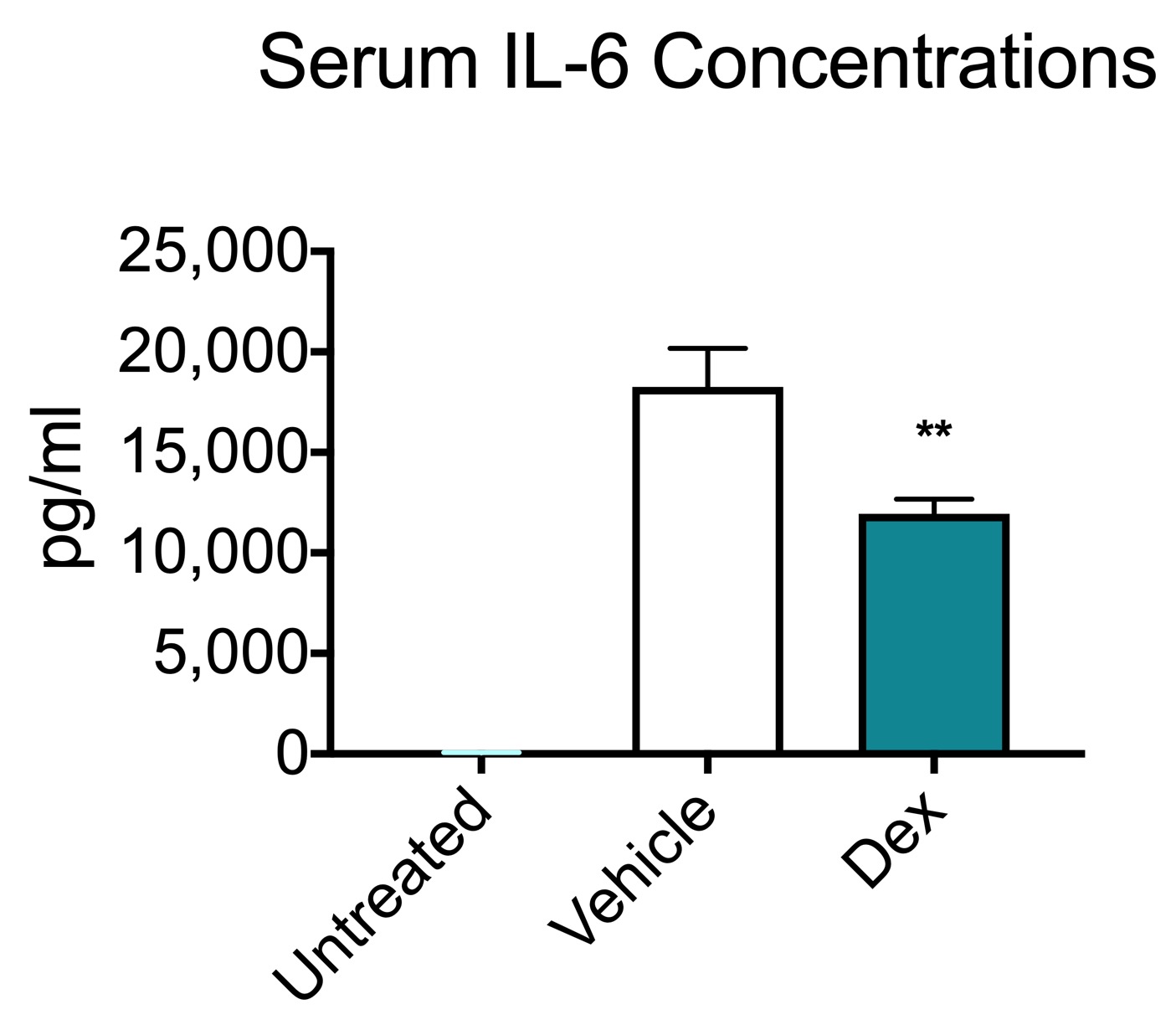
LPS model of sepsis serum cytokine data. Following a bolus injection of LPS, blood was collected from animals and serum was isolated. LPS injection induced a significant increase in TNF-⍺ and IL-6 in vehicle-treated animals. Oral administration of dexamethasone, a potent anti-inflammatory, significantly attenuated the both TNF-⍺ and IL-6 indicating a prevention of the inflammation response. These results are similar to literature reports (Ochalski et al., 1993; Zuckerman et al., 1989). Data are mean serum concentrations ± SEM; **p<0.01; *** p< 0.001 (n=6).
The mouse LPS model of sepsis is typically run as an acute model (one day study) with a single administration of test article. In addition to monitoring TNF-α levels the evaluation of other cytokines and biomarkers such as corticosterone and often incorporated into the studies.
References
- Ochalski, SJ, Hartman, DA, Belfast, MT, Walter, TL, Glaser, KB, and Carlson, RP. Inhibition of endotoxin-induced hypothermia and serum TNF-alpha levels in CD-1 mice by various pharmacological agents. Agents Actions. 1993:39.
- Zuckerman, SH and Bendele, AM. Regulation of serum tumor necrosis factor in glucocorticoid-insensitive and –resistant endotoxin shock models. Infect. Immun. 1989 Oct;57(10):3009-13.
