Influenza Pulmonary Disease Model
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Influenza is a viral disease that is responsible for the death of over 30,000 individuals in the U.S each year. One of the major causes of death from this virus is from pulmonary complications—both direct, as from pulmonary extravasation induced by viral infection, and indirect, from secondary infection which occurs following viral induced pneumonia. Therefore, this remains a disease with significant unmet medical need. Melior has established an influenza mouse model which faithfully recapitulates the disease progression as it occurs in humans. Accordingly, it can be used to evaluate different classes of therapeutics such as antivirals, anti-inflammatories, and pulmonary therapeutics. Insofar as this is a faithful model of viral-induced cytokine storm, with a resultant pulmonary edema and acute respiratory distress syndrome (ARDS) this influenza mouse model provides a sound basis to study therapeutics for pandemic diseases such as COVID-19, MERS and SARS.
The study outlined below illustrates the therapeutic effect of the influenza antiviral, oseltamivir (Tamiflu®; a neuraminidase inhibitor), which reduces reduces the viral load by interfering with virion formation. The figure below also illustrates the means by which an influenza study can be configured, depending upon test article mechanism of action and study objectives, to either a lethal of sublethal configuration. Oseltamivir significantly reduces mortality and improves the rate of recovery in mice infected with the influenza virus.
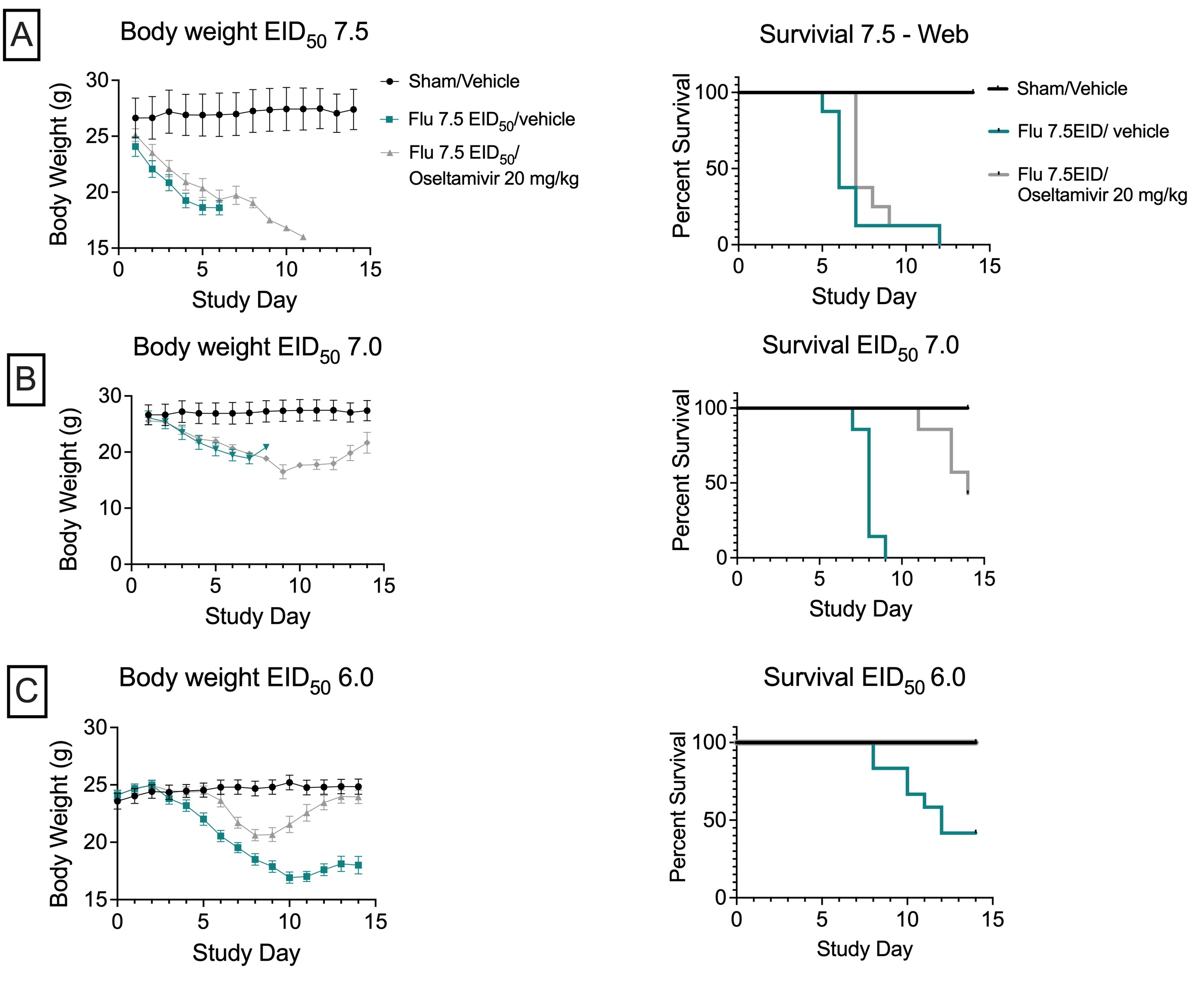
Influenza mouse model. Study was conducted in C57Bl/6J male mice, age 6-7 weeks. Mice were inoculated with influenza virus H1N1 A/PR/8/34 at a dose of either (A) 7.5Lg EID50* (B) 7.0 Lg EID50* or (C) 6.0 Lg EID50*. On the same day as inoculation, treatment was started as either vehicle or oseltamivir (20 mg/kg PO q.d.). Oseltamivir exhibits significant protection towards survival (BW data are mean ± SEM; n=8). * embryonic infectious dose for 50% of chick embryos.
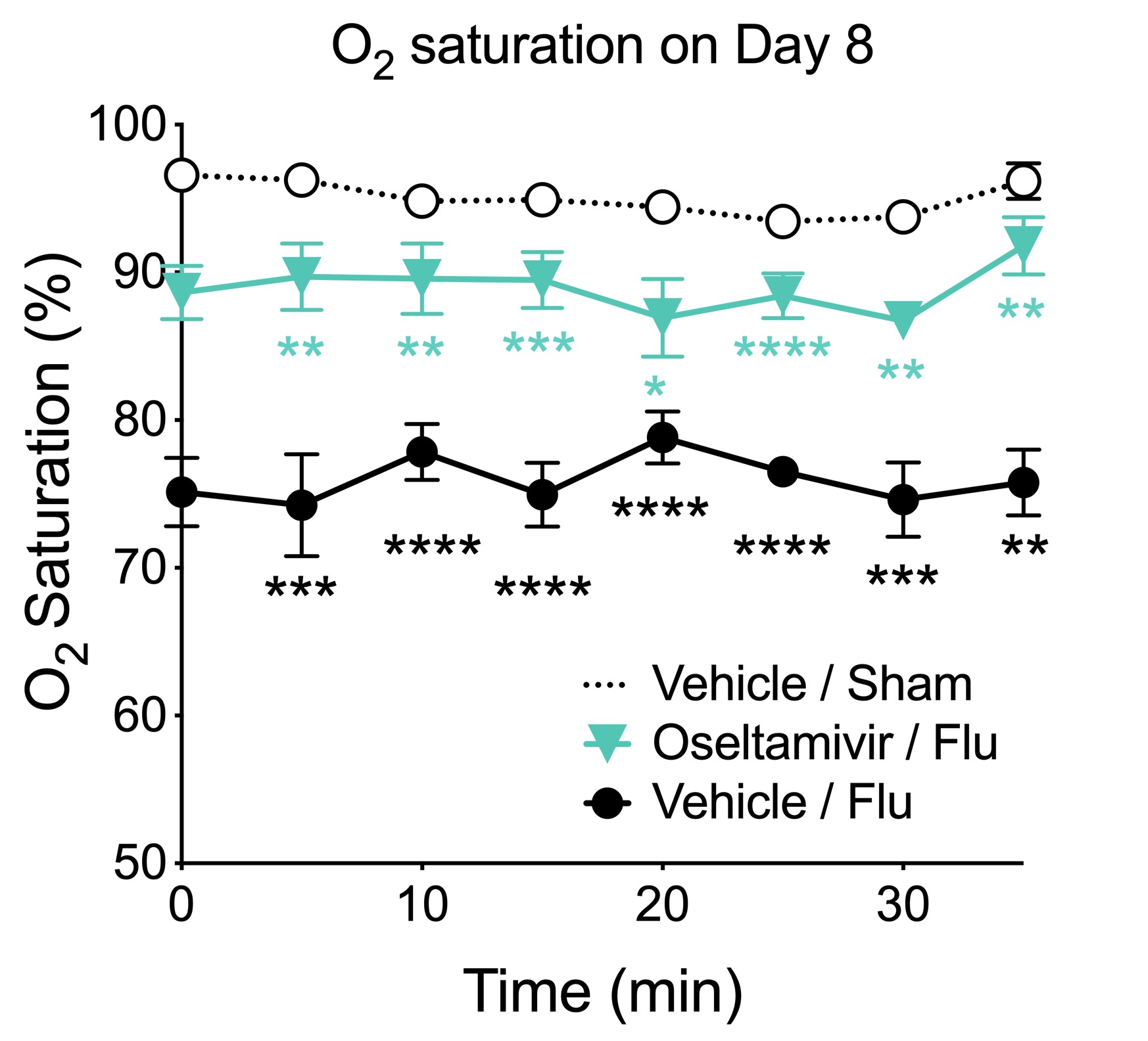
Blood oxygen saturation (SpO2). Blood oxygen saturation is measured by pulse oximetry. This figure shows that SpO2 is significantly decreased in influenza-infected animals compared to uninfected controls. Oseltamivir treatment significantly improves SpO2 compared to vehicle-treated animals. (Data are mean ± SEM; * p< 0.05, ** p<0.01, *** p<0.001, **** p<0.0001, influenza-infected / vehicle-treated are compared to sham(denoted in black); influenza-infected oseltamivir-treated are compared to influenza-infected / vehicle-treated (denoted in blue); n=8).
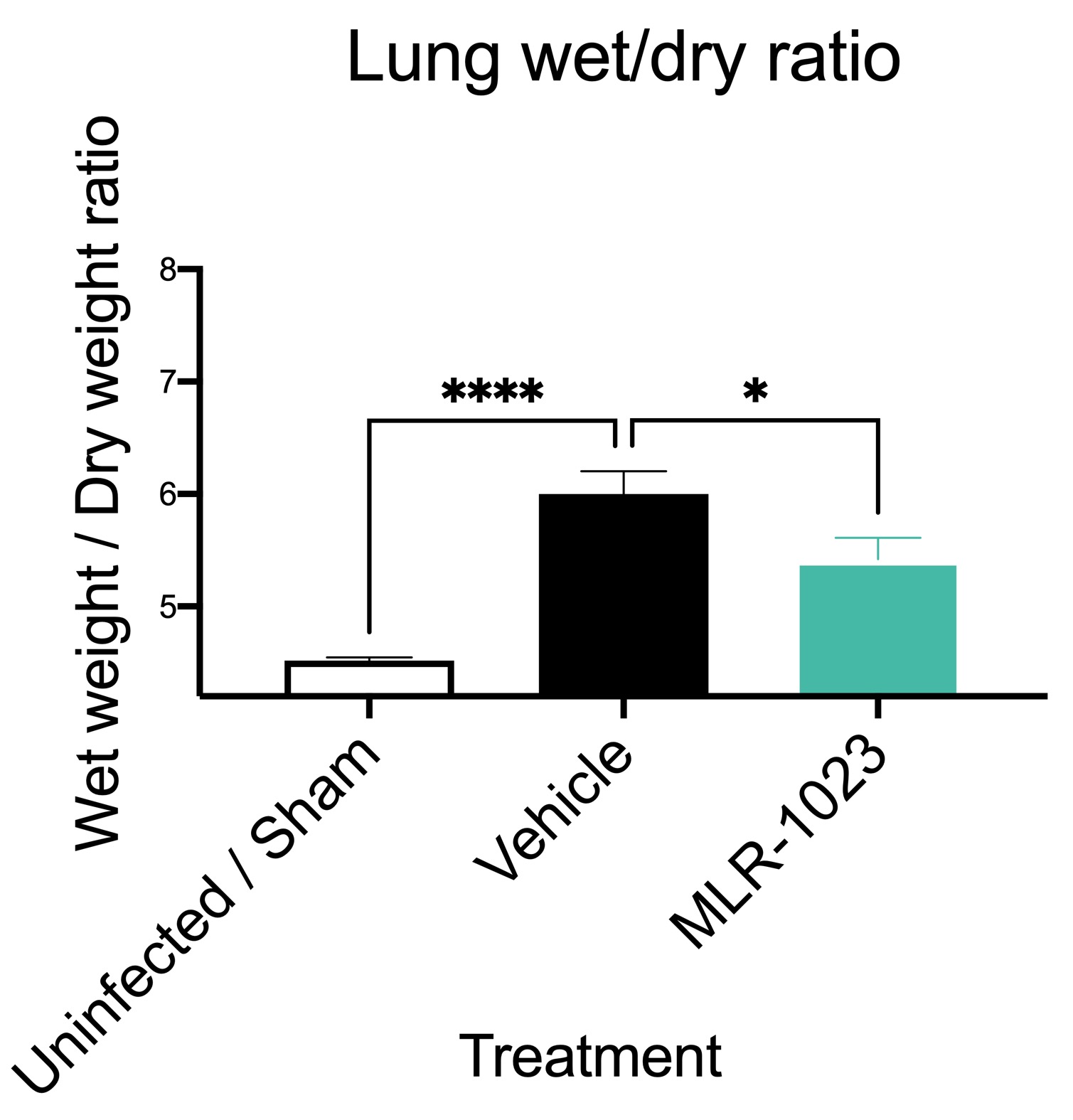
Pulmonary extravasation (edema). Influenza infection is associated with plasma fluid leaking into the lung (extravasation). This can be measured by wet weight / dry weight ratio. The figure shows significantly increase lung edema in flu-infected animals compared to uninfected animals. Edema is significantly reduced by pretreatment of animals with the pulmonary therapeutic candidate MLR-1023 (Data are mean ± SEM; n=8; * p< 0.05, **** p<0.0001).
The influenza mouse model is typically a 7-14-day study. In addition to measures survival and body weight as shown here it is also appropriate to measure parameters of pulmonary of edema (wet weight / dry weight ration or Evan’s Blue extravasation), viral load, measures of cytokines such as IL-6, TNF-α, and MCP-1 and / or lung histology



 Interested in running an Influenza study?
Interested in running an Influenza study?