Respiratory Depression Assay
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Respiratory depression is a significant side effect associated with opioid treatment of acute and chronic pain. Efforts to generate analgesics that dissociate pain relief from negative side effects are critical. Therefore many analgesic test article characterization studies incorporate respiratory depression as a measured endpoint.
Pulse oximetry is a relatively simple and noninvasive technology for monitoring heart rate, oxygen saturation, and breathing rate in rodents. The method involves temporarily harnessing animals with a special collar that emits a beam of dual-wavelength light with a sensitive photodetector. Oxygen saturation is particularly reliable parameter for evaluating respiratory depression.
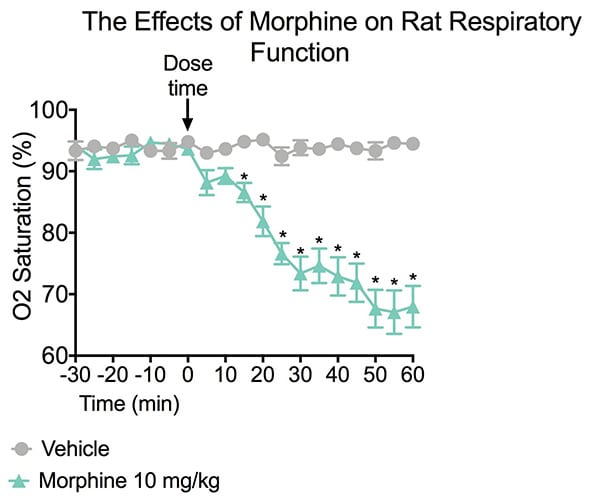
The study summarized below examines the effects of an opioid in a respiratory depression study using pulse oximetry. Blood oxygen saturation levels were assessed in conscious rats. Prior to dosing a 30 minute baseline was established for each animal. After dosing, a 5-second average of the oxygen saturation value was sampled every 5 minutes for 60 minutes.
A significant reduction in O2 saturation was found in rats treated with MORPH compared to rats treated with vehicle. Data are mean ± SEM; **p<0.01 compared to vehicle (N=8).
The rodent pulse oximetry procedure to measure Respiratory Depression is most typically at least a 3-day procedure to account for 2 acclimation days where the animals are fitted with the pulse oximetry collars for about 1 hour periods. This is a non-invasive procedure that lends itself well to incorporating at several points over the course of a chronic model or treatment regimen.



 Interested in running a Respiratory Depression study?
Interested in running a Respiratory Depression study?