Kidney Xenograft Model - HEK293
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Renal cell carcinoma (RCC) is the most common type of kidney cancer, accounting for about 2-3% of all adult malignancies and approximately 90-95% of all kidney cancers. The incidence of RCC has been increasing in recent years, with an estimated 76,080 new cases and 13,780 deaths from RCC in the United States in 2022.
Treatment options for RCC depend on the stage and location of the cancer, as well as the patient’s overall health. The standard treatment for localized RCC is surgical removal of the tumor, with partial or radical nephrectomy being the most common procedures. For metastatic RCC, there are several treatment options, including targeted therapy, immunotherapy, and chemotherapy. Targeted therapy drugs, such as tyrosine kinase inhibitors (TKIs) and mTOR inhibitors, have revolutionized the management of metastatic RCC in the past decade. Immunotherapy, such as immune checkpoint inhibitors, has also shown promising results in the treatment of RCC.
Despite these advances, the prognosis for patients with advanced and metastatic kidney cancer remains poor, with a 5-year survival rate of less than 10%. There is a need for new therapies that can improve survival and quality of life for these patients. Melior’s kidney cancer model is an important tool towards this end.
HEK293 cells are a type of human embryonic kidney cell line that was first isolated in 1977 from primary cultures of human embryonic kidney cells transformed with sheared human adenovirus type 5 DNA. While this cell line is not a direct model of any specific type of kidney cancer, due to their origin from kidney tissue, they have been used as a tool for studying various types of kidney cancer, including RCC. In particular, HEK293 cells have been used as a model system to study the molecular mechanisms underlying kidney cancer, and to test the efficacy of potential therapeutic agents against RCC cells.
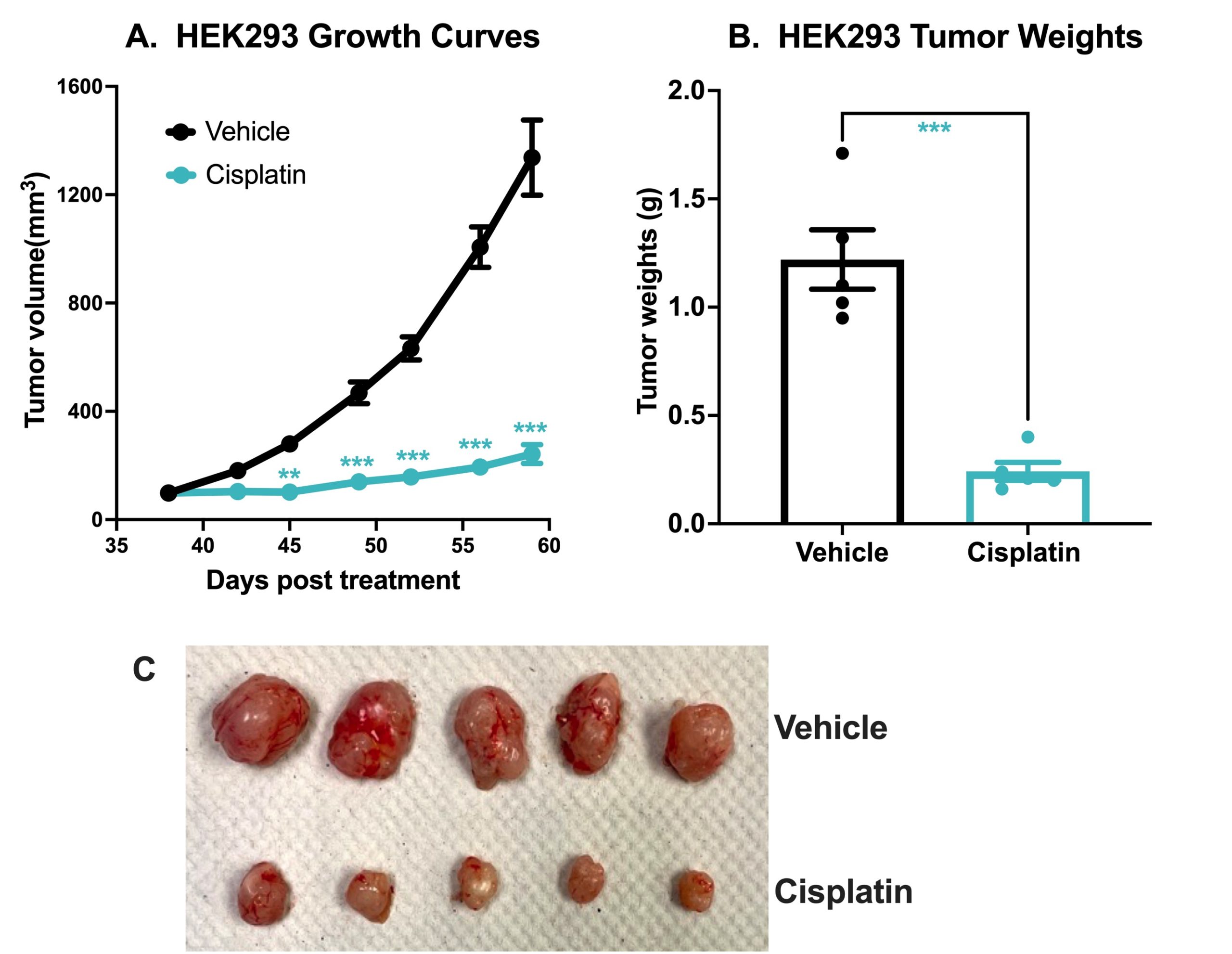
Chemotherapeutic Validation of a HEK293 Xenograft Model. 7 x106 HEK293 cells were subcutaneously injected into the rear flank of nude mice. Once the tumor size reached ~100mm3 (Day 38), mice were randomized into vehicle control group (treated with normal saline) and cisplatin group (3 mg/kg, IP once/week ). Tumor volume was monitored twice per week using calipers (A). At the end of the study (Day 59), animals were sacrificed, tumors excised and weighed (B,C). Data are mean ± SEM; n=5 /group; ** p<0.01, *** p<0.001 by Student’s t-test.
Melior can initiate your HEK293 tumor model study with very short lead times and with bespoke study design to suit your needs. Including time to establish tumor-bearing mice (5-6 weeks) and typical treatment times (3-4-weeks) these studies normally run for approximately 10 weeks..
References
American Cancer Society. Cancer Facts & Figures 2022. Atlanta: American Cancer Society; 2022
Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ. 2014; 349:g4797
Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017; 3:17009
Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36(1):59-72