Cell Line-Derived Xenograft Models
Predict the success of your novel cancer therapy with our cell line-derived xenograft models.
Choosing the cell line-derived xenograft (CDX) mouse model that best fits your pharmaceutical R&D is quick and simple when you leverage our extensive resources and deep knowledge of in vivo modeling.
Evaluate oncology therapies against human cells—early and reliably
CDX mouse models implanted with well-characterized cancer cell lines offer a consistent tumor phenotype and provide the early opportunity to assess your novel drugs against human cells.
With their ease of use and cost-effectiveness, CDX models are a reliable screening tool—ensuring that you invest in the most promising candidates.
Advantages of choosing a CDX mouse model
- Assess with confidence: Get reliable, reproducible data when you work with a CDX model derived from established cancer cell lines.
- Get species-specific evaluation data: Enhance your understanding of species-specific drug interactions with easy access to human cancer cells.
- Streamline your preclinical testing: Screen new therapies using CDX mouse models and complementary syngeneic models for a seamless R&D experience.
Elevate your preclinical research with our validated CDX mouse models
Explore the potential of your novel therapeutic in any of our CDX mouse models to gain early insights into how your cancer drug behaves in its intended target species.
Ready to take your cancer therapy further with custom studies?
Contact us for more information on our additional and bespoke services including IVIS imaging, PK studies, and metabolic analyses.
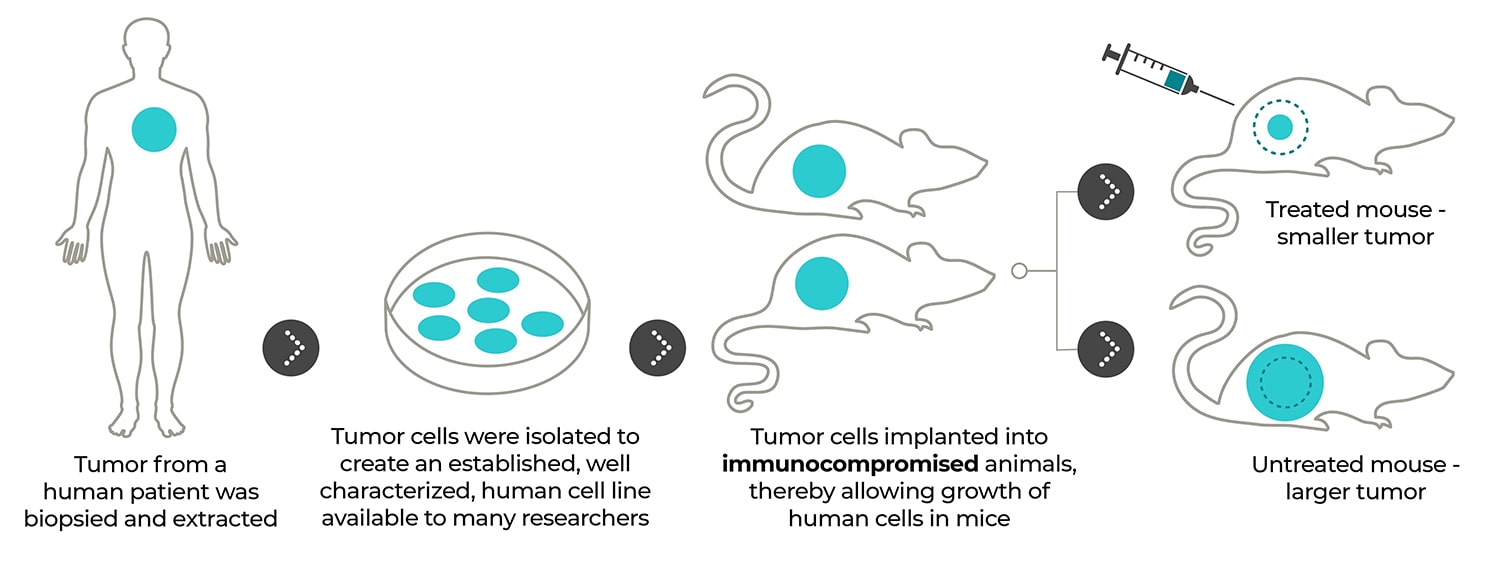
Cell line-derived xenograft models: from tumor to cell culture to mice. The generation of CDX mouse models starts with the selection of an established human cell line of interest. The human tumor cells are implanted into immunocompromised mice where they grow and serve as an excellent model for chemotherapeutic R&D testing.
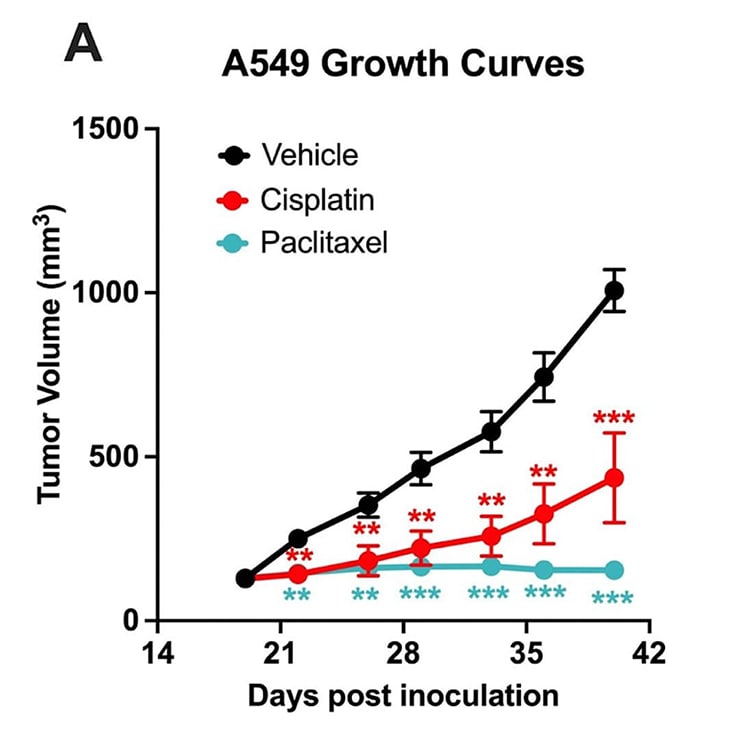
Chemotherapy validation in human lung cancer A549 xenograft model. 5 x106 A549 cells were subcutaneously injected into the rear flank of nude mice. Once the tumor size reached ~100mm3 (Day19), mice were randomized into vehicle control group (treated with normal saline), paclitaxel (20 mg/kg, IP twice/week), and cisplatin group (3 mg/kg, IP twice/week). Tumor volume was monitored twice per week using calipers. Data are mean ± SEM; n=5 /group; ** P<0.01, *** P<0.001 by Student’s t-test.
Did you know you could save time and money in your cancer R&D?
CDX models offer a cost-effective and time-efficient way to screen potential anticancer drugs, enabling researchers to quickly evaluate the efficacy of a wide range of therapeutic compounds against various cancer types.
Publications
Discover the impactful research that our products are facilitating. This selection of publications, authored by researchers like you, underscores our commitment to advancing cancer research and development.
Frequently Asked Questions
CDX mice are useful for achieving initial drug candidate screening and early validation studies that are consistent and reliable. Owing to the standardized nature of cell lines, these experiments can be highly reproducible, easy to use, and cost-effective. However, their simplicity is not suited to all pharmaceutical R&D applications. CDX models lack a fully functioning immune system, limiting their use in evaluating immunotherapies, for this researchers may explore syngeneic models or PDX models in humanized mice. Different models are suited to various applications and depend on your research goals. Working with experts to choose the right model for you is the best course of action toward success.
Patient-derived xenograft (PDX) models are excellent at retaining the tumor heterogeneity of the patient’s original tumor, making them more representative of clinical scenarios. Our PDX models are suitable for use in humanized mice, enabling R&D scientists to study important tumor-immune system interactions. To better evaluate your novel immunotherapies, you can also explore our syngeneic tumor models that enable the study of treatment responses within an intact immune system. Our syngeneic mice provide the perfect solution for studying immune therapies such as CAR-T or checkpoint inhibitors. With complementary tumor types across our modeling options, we have something for every oncology R&D need.
Patient-derived xenograft (PDX) models are created by implanting human tissue directly from patients into immunodeficient mice. This approach preserves the tumor’s heterogeneity and offers high clinical relevance for oncology R&D. Whereas cell line-derived xenograft (CDX) models involve implanting established human cancer cell lines into immunodeficient mice, providing consistency and reproducibility for high-throughput drug screening.