The MCF-7 Xenograft Model for Breast Cancer
Validate your novel therapeutics with the most-studied human breast cancer cells in vivo.
1 in 8 women get a diagnosis of breast cancer at some point in their lifetime. 80-85% of all breast tumors are hormone-receptor or HER2 positive, making the development of chemotherapies that work with hormone receptor expression an essential effort in combatting breast cancer.
The MCF-7 xenograft mouse model is the ideal in vivo model for testing your best-performing in vitro breast cancer chemotherapy candidates in the presence of human estrogen and progesterone receptors.
50 years of research in one reliable model
Originating from a 69-year-old woman’s metastatic adenocarcinoma in 1973, MCF-7 cells have become the most commonly used cell line for breast cancer research around the globe. Due to their expression of estrogen and progesterone receptors, MCF-7 cells are an ideal screening tool for developing chemotherapies in the presence of hormonal regulation, which may affect responsiveness to breast cancer therapies. Implanting these well-established cells into mice offers a realistic environment for testing novel breast cancer drugs.
Although cells are inexpensive and relatively easy to work with, they do not accurately model the in vivo environment. The MCF-7 xenograft mouse model allows you to test your novel cancer drug in a complex biological environment, helping you better understand your drug’s efficacy.
Fast-track your candidate to the clinic by monitoring its efficacy with ease in a well-established cell line in vivo.
Advantages of the MCF-7 xenograft model
- Visualize breast tumors easily with firefly luciferase (Luc2)
- Work with a widely-used cell line in an in vivo environment.
- Speed up your experiments and get high-quality results fast.
- Translate your results to the clinic with a human cell line xenograft.
Assess your breast cancer drug quickly and easily with the MCF-7-Luc2 xenograft model
The MCF-7-Luc2 xenograft model is a derivative of the MCF-7 cell line. It retains the same stability, reliability, and track record but has been engineered to express the luciferase 2 (Luc2) gene.
Luc2 is a bioluminescent reporter used frequently in molecular biology for live tracking –
non-invasively monitoring cells in real time using IVIS and other bioluminescence imaging techniques. You can use these techniques to monitor the tumor growth, metastasis, or a tumor’s response to treatment within the MCF-7-Luc2 xenograft model at multiple time points without sacrificing the animal.
Ready to enrich your cancer R&D with custom-integrated studies?
Whether you are trying to validate a breast cancer drug candidate in vivo or assess the activity of tumor cells in a 3D environment, our MCF-7 xenograft model can be prepared within 1 to 2 weeks, and results will be available after 6 weeks of treatment.
Contact us for more information on our additional and bespoke services, including IVIS imaging, PK studies, and metabolic analyses.
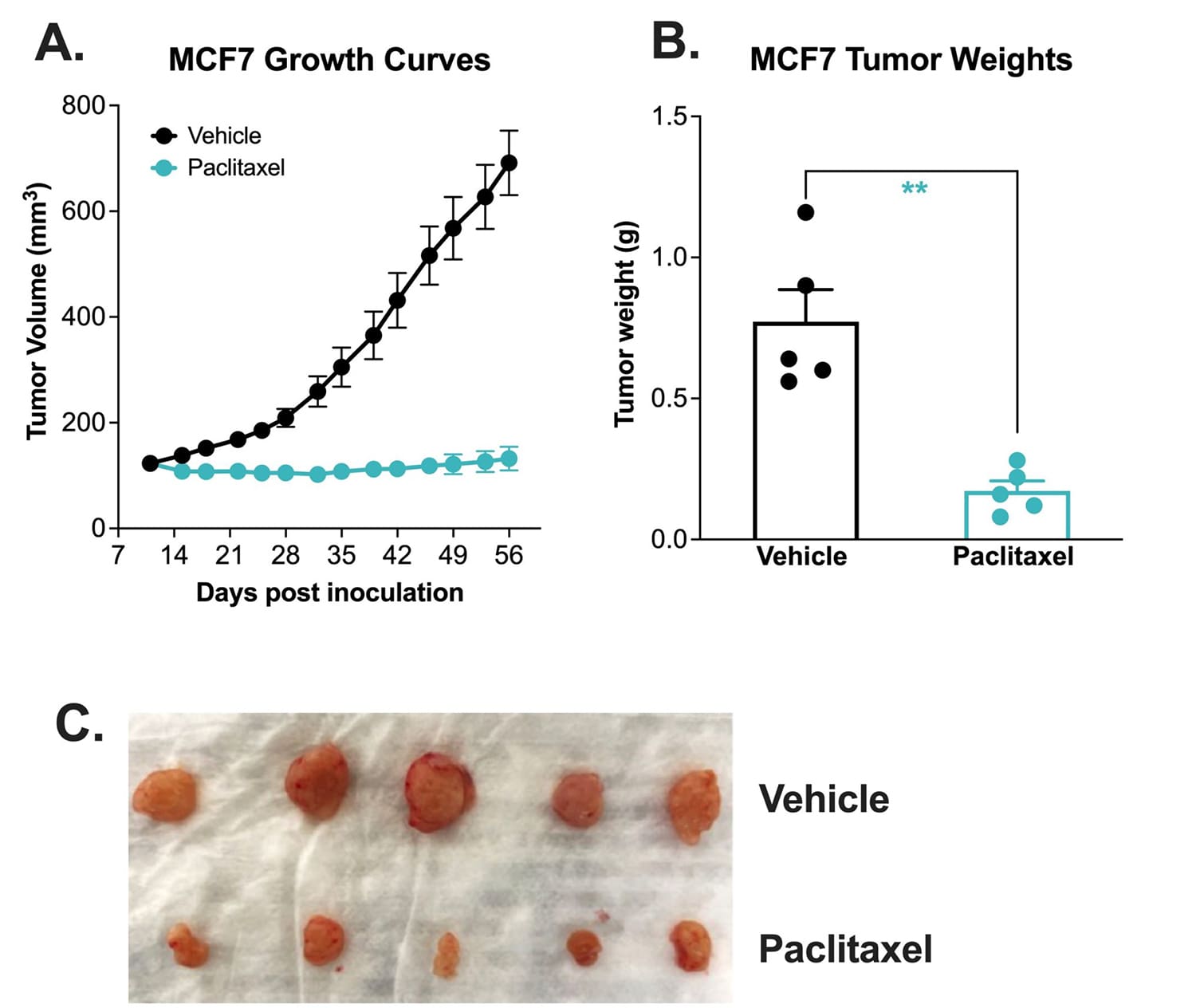
Chemotherapy validation of human breast heterotopic MCF-7 xenograft model.
The MCF-7 tumor model was created by subcutaneously injecting 1 x107 MCF-7 cells (in Matrigel) into the rear flank of the nude mice. Once the tumor size reached ~100mm3 (Day 11), mice were randomized into groups and treated with vehicle control (normal saline) or paclitaxel (20 mg/kg, IP once/week). Tumor volume was monitored twice per week using calipers (A). At the end of the study (Day 56), animals were sacrificed, tumors were excised and weighed (B, C). Data area mean ± SEM. n=5/group. * P<0.05, ** P<0.01 *** P<0.001 by Student’s t-test.
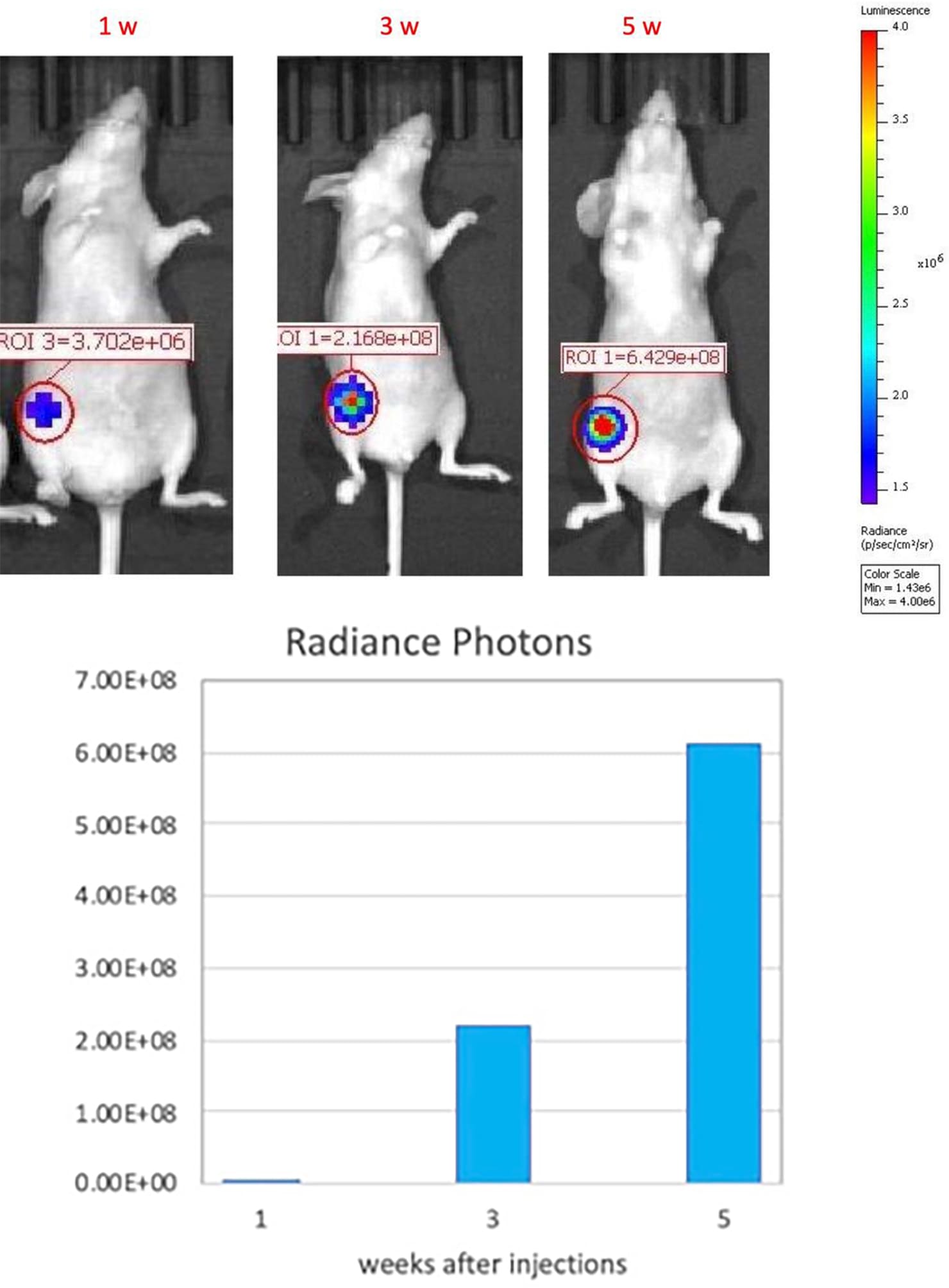
Growth kinetic monitoring of human orthotopic breast tumors in an MCF-7-Luc2 xenograft model. The orthotopic MCF-7 xenograft tumor model was created by orthotopically injecting 1 x106 MCF-7-Luc2 (an MCF-7 cell line that carries a stable insertion of the firefly luciferase gene) cells (in Matrigel) into the mammary fat pad of athymic nude mice(nu/J). Tumor growth was monitored at 1,3, and 5 weeks after injection by IVIS imaging system.
Did you know the MCF-7 xenograft model is complementary to our syngeneic EMT-6 model?
Melior makes syngeneic breast cancer mouse models that use mouse rather than human breast cancer cell lines. This EMT-6 syngeneic model complements Melior’s human xenograft breast cancer models. By comparison, the MCF-7 is an ER+, PR+, HER2- while EMT-6 is ER-, PR-, HER2-.
Publications
Behzadi, R., Ahmadpour, S., Amiri, F. T., Kavosian, S., Asori, M., & Hosseinimehr, S. J. (2023). Choosing the Right Protocol to Establish MCF-7 Tumor Xenograft in Nude Mice. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 23(2), 222-226.
Detre, S., Riddler, S., Salter, J., A’Hern, R., Dowsett, M., & Johnston, S. R. (2003). Comparison of the selective estrogen receptor modulator arzoxifene (LY353381) with tamoxifen on tumor growth and biomarker expression in an MCF-7 human breast cancer xenograft model. Cancer research, 63(19), 6516-6522.
Divisova, J., Kuiatse, I., Lazard, Z., Weiss, H., Vreeland, F., Hadsell, D. L., … & Lee, A. V. (2006). The growth hormone receptor antagonist pegvisomant blocks both mammary gland development and MCF-7 breast cancer xenograft growth. Breast cancer research and treatment, 98, 315-327.
Johnston, S. R., Boeddinghaus, I. M., Riddler, S., Haynes, B. P., Hardcastle, I. R., Rowlands, M., … & Dowsett, M. (1999). Idoxifene antagonizes estradiol-dependent MCF-7 breast cancer xenograft growth through sustained induction of apoptosis. Cancer research, 59(15), 3646-3651.
Ramos, G., Loperena, Y., Ortiz, G., Reyes, F., Szeto, A., Vera, J., … & Washington, A. V. (2014). The addition of a pregnenolone pendant group enhances the anticancer properties of titanocene dichloride in a mcf-7 xenograft model. Anticancer research, 34(4), 1609-1615.
Frequently Asked Questions
There is no single ‘best’ mouse model of breast cancer. It depends on what type or stage of cancer you are trying to model. The MCF-7 xenograft model is ideal if you aim to target hormone receptor-expressing breast tumors. If you are studying triple-negative breast cancer, you may be interested in complementing your xenograft studies with the compatible EMT-6 model.
MCF-7 is estrogen receptor-positive (ER+), progesterone receptor-positive (PR+), and human epidermal growth factor receptor 2-negative (HER2-). This cell line has historically been a popular choice to test drug candidates that target hormone receptors.
Syngeneic tumor models, derived from cancerous mouse tissue and grown inside a mouse, are helpful in some cases but are less readily translatable to the clinic than xenograft models, which use tumors derived from human cells. Because xenograft tumors can come from the cells of any species, they also offer more variety in the types of models that can be developed.
Citations
Almansour, Nahlah Makki. “Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence.” Frontiers in molecular biosciences vol. 9 836417. 25 Jan. 2022, doi:10.3389/fmolb.2022.836417
National Breast Cancer Foundation. “What is Breast Cancer?” National Breast Cancer Fountation, Inc. 2024. Retrieved March 7, 2024 from: https://www.nationalbreastcancer.org/what-is-breast-cancer/?&utm_source=google&utm_medium=cpc&utm_campaign=85386326&utm_term=breastcancer&gad_source=1&gclid=CjwKCAiAloavBhBOEiwAbtAJO2I5LuJPdjCs4mApVhyU49eTor79kp7iQWU_eKp5lpZyIi6nh3ttmhoCarwQAvD_BwE
Raymond Reilly, Breast Cancer, Editor(s): S.J. Enna, David B. Bylund, xPharm: The Comprehensive Pharmacology Reference, Elsevier, 2007, Pages 1-9, ISBN 9780080552323, https://doi.org/10.1016/B978-008055232-3.60809-8..





