Human Prostate Xenograft Model /LNCaP
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Prostate cancer is the second most common cancer in men worldwide and the fifth leading cause of cancer death in men. The incidence of prostate cancer varies widely by geographic region, with the highest rates in North America, Australia, and Europe, and the lowest rates in Asia and Africa. Age is a significant risk factor for prostate cancer, with most cases occurring in men over the age of 50.
There are several advanced treatment options available for those with the disease, including surgery, radiation therapy, hormone therapy, chemotherapy, immunotherapy, cryotherapy, and High-Intensity Focused Ultrasound (HIFU). Although there are many treatment options available for men with advanced prostate cancer, the disease remains incurable once it has metastasized beyond the prostate gland. New and more effective treatments are needed to prolong survival and improve quality of life for men with advanced prostate cancer. Melior’s model of prostate cancer is an important tool towards this goal.
LNCaP cells are a commonly used model of prostate cancer originally derived in 1977 from a lymph node biopsy of a 50-year-old Caucasian male with metastatic prostate cancer. They are an androgen-sensitive human prostate adenocarcinoma cell line that has a slow growth rate, and are widely used in prostate cancer research due to their ability to recapitulate many aspects of human prostate cancer.
LNCaP cells express the androgen receptor (AR) and require androgens for growth and survival. The cells are typically grown in culture medium containing fetal bovine serum, which contains small amounts of androgens. Androgens activate the AR on LNCaP cells and upregulate downstream signaling pathways that promote cell proliferation and survival. LNCaP cells are therefore used as a model to study the mechanisms of androgen signaling in prostate cancer, and as a screening tool for potential therapies that target the androgen receptor signaling pathway.
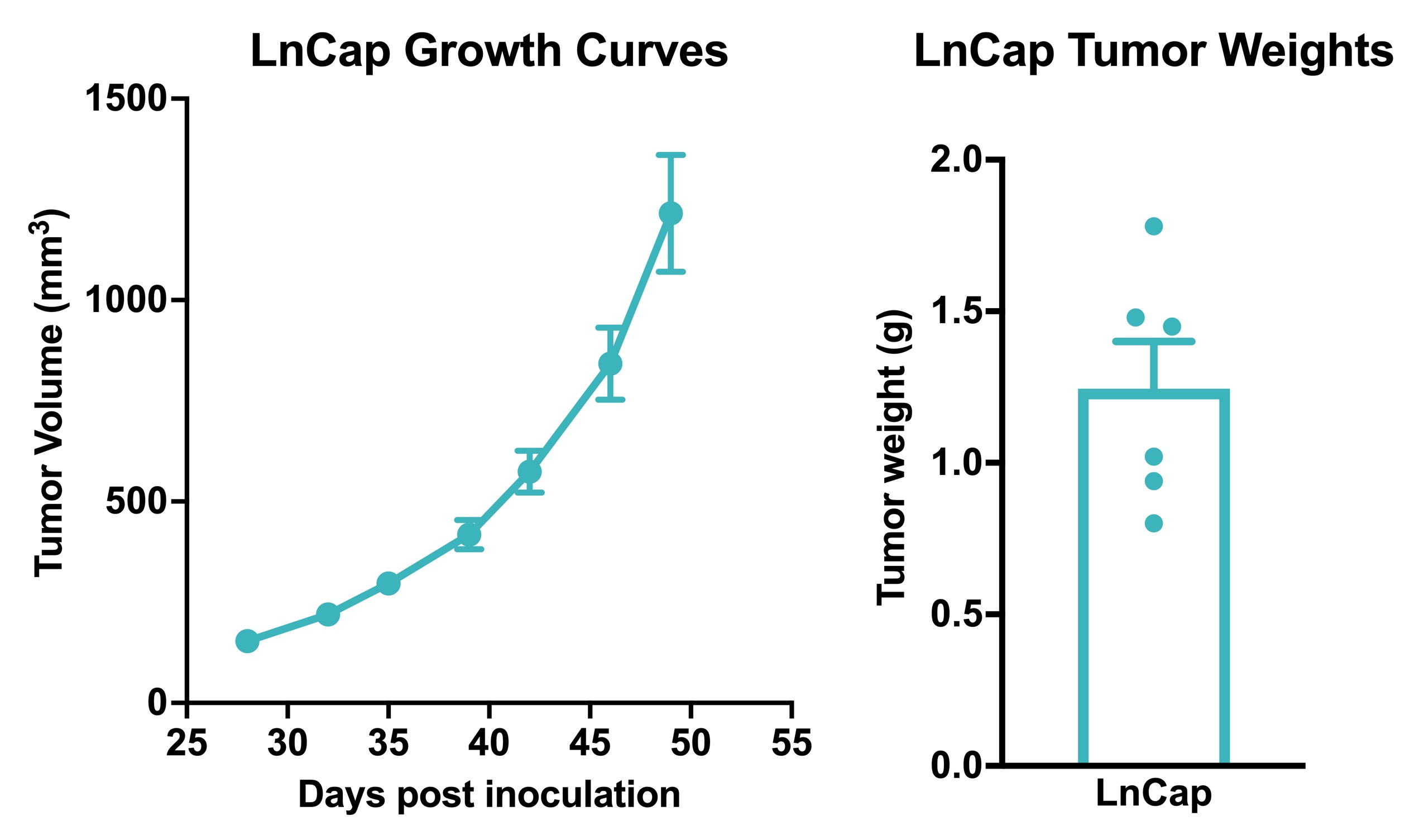
Human prostate cancer LnCaP xenograft model. 1×106 LnCaP cells were subcutaneously injected into the rear flank of nude mice. The growth of tumor was monitored twice per week. Data area mean +/- SEM.
Melior can initiate your LNCaP tumor model study with very short lead times and with bespoke study design to suit your needs. Including time to establish tumor-bearing mice (3-5 weeks) and typical treatment times (3-4-weeks) these studies normally run for approximately 8-10 weeks.
References
- Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK. The LNCaP cell line–a new model for studies on human prostatic carcinoma. The Prostate. 1983;14(4):395-406. doi: 10.1002/pros.2990140409.
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nature Medicine. 2004;10(1):33-39. doi: 10.1038/nm972.
- Wang X, Yu J, Sreekumar A, Varambally S, Shen R, Giacherio D, Mehra R, Montie JE, Pienta KJ, Sanda MG, et al. Autoantibody signatures in prostate cancer. The New England Journal of Medicine. 2005;353(12):1224-1235. doi: 10.1056/NEJMoa051931.



 Interested in running a human prostate tumor model study?
Interested in running a human prostate tumor model study?