Human Glioblastoma Xenograft Model / U87 MG
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Gliomas are a group of primary brain tumors that arise from glial cells and represent about 27% of all primary brain and central nervous system tumors, making them the most common type of primary brain tumor in adults. Glioblastomas are the most common and aggressive type of glioma accounting for about 15% of all brain tumors and about 50% of all gliomas.
Treatment for gliomas and glioblastomas typically involves surgery, radiation therapy, and chemotherapy, although the specific treatment plan depends on the location and size of the tumor, as well as the patient’s overall health.
The most recent advances in treatment options include the use of immune checkpoint inhibitors (ICIs) which work by removing the brake on the immune system, allowing it to more effectively target and destroy cancer cells. Currently, there are several clinical trials underway to test the efficacy of ICIs in combination with other therapies such as radiation and chemotherapy. Several targeted therapies have been developed that aim to inhibit specific molecular pathways that drive glioblastoma growth. One such example is bevacizumab, which targets the VEGF pathway to prevent blood vessel formation and disrupt the tumor’s nutrient supply. However, clinical trials have produced mixed results with bevacizumab and it is not approved for use in all countries.
Despite advances in treatment, glioblastomas remain one of the most difficult types of cancer to treat, and the prognosis is generally poor. The current median survival time for patients with glioblastomas is about 12-15 months, and the five-year survival rate is less than 10%. There is a high unmet medical need for better glioblastoma treatment options. Melior’s animal model of glioblastoma is an important tool towards this goal.
The U87 MG cell line is a widely used model of glioblastoma that was originally derived from a human glioblastoma tumor and is characterized by a number of features that make it a useful model of glioblastoma. These include the ability to form tumors in immunodeficient mice, a high degree of invasiveness, and the expression of genes and proteins that are commonly found in human glioblastomas. Molecular alterations include mutations in the TP53 and PTEN genes and amplification of the EGFR gene. U87 MG cells are sensitive to a number of chemotherapeutic agents and targeted therapies, including temozolomide and bevacizumab.
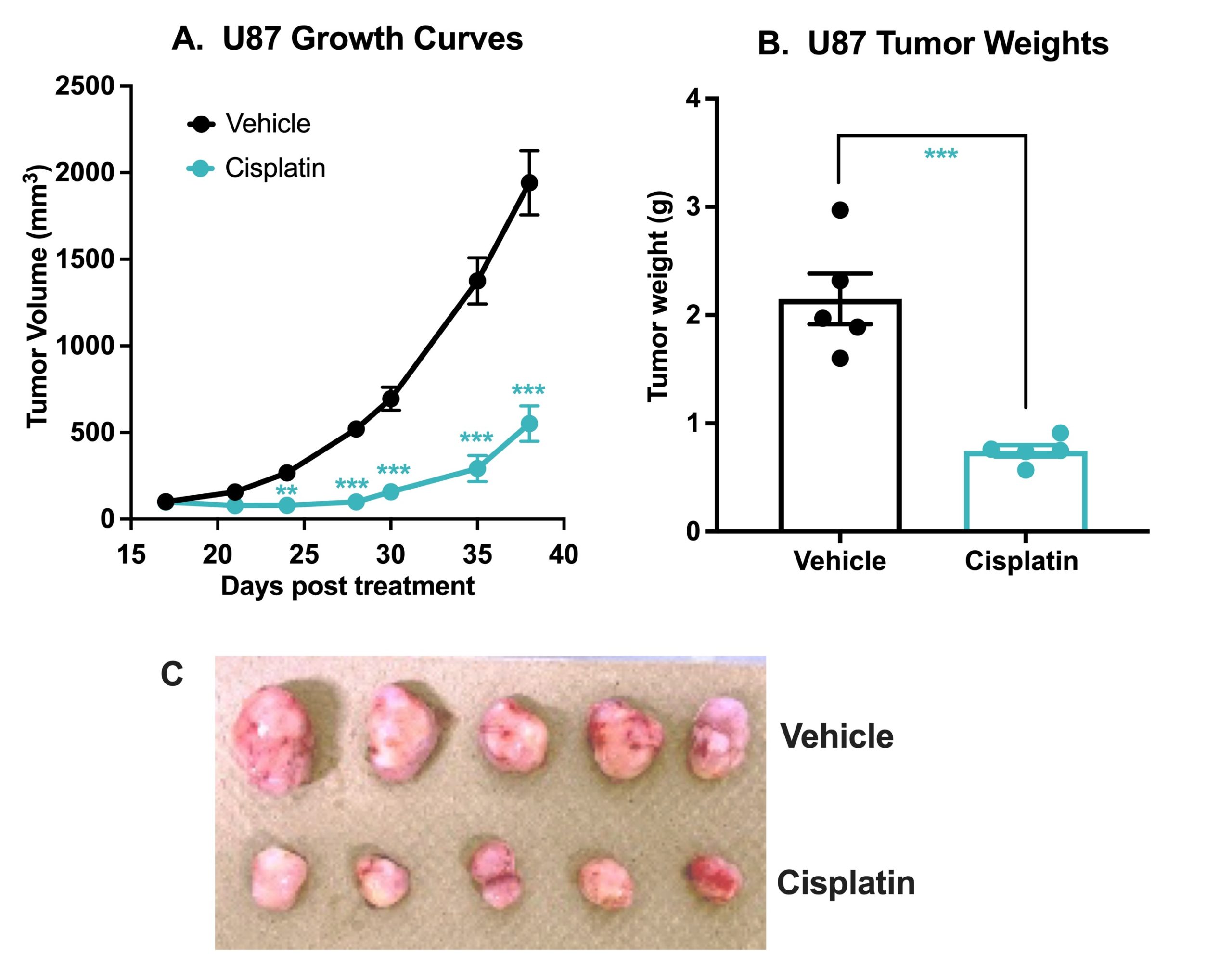
Chemotherapy validation in human glioma U87 MG subcutaneous xenograft model. 1 x106 U87 MG cells were subcutaneously injected into the rear flank of nude mice. Once the tumor size reached ~100mm3 (Day 17), mice were randomized into vehicle control group (treated with normal saline) and cisplatin group (3 mg/kg, IP once/week ). Tumor volume was monitored twice per week using calipers (A). At the end of the study (Day 38), animals were sacrificed, tumors excised and weighted (B,C). Data are mean ± SEM; n=5 /group; **p<0.01, *** p<0.001 by Student’s t-test.
Melior can initiate your U87 MG tumor model study with very short lead times and with bespoke study design to suit your needs. Including time to establish tumor-bearing mice (2-3 weeks) and typical treatment times (3-4-weeks) these studies normally run for approximately 6-7 weeks..
References.
- Ostrom QT, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2009-2013. Neuro-oncology. 2016;18(suppl_5):v1-v75.
- Louis DN, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-oncology. 2021;23(8):1231-1251



 Interested in running a glioblastoma xenograft model study?
Interested in running a glioblastoma xenograft model study?