Hepa 1-6 Syngeneic Model of Liver Cancer
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
By far the most common form of liver cancer is hepatocellular carcinoma (HCC). HCC is a particularly difficult to treat and has a high mortality rate despite advances in HCC research. The majority of HCC cases are diagnosed at an advanced stage, often after it has metastasized to other organs, and treatment options for such advanced HCC are limited. HCC is a highly heterogeneous disease, and treatment responses can vary widely between patients. Personalized treatment approaches that take into account the genetic and molecular characteristics of each patient’s tumor may improve outcomes. HCC has a high rate of recurrence even after successful treatment, and there is a need for more effective strategies to prevent recurrence and improve long-term outcomes for patients. Therefore, there is a need for more effective and targeted therapies that can improve outcomes for patients with advanced HCC. Melior’s animal models of HCC are an important tool towards this goal.
Melior’s mouse-derived Hepa 1-6 syngeneic liver tumor model complements our human derived HepG2 xenograft liver tumor model. Hepa 1-6 cells were derived from a hepatoma that developed spontaneously in a male C57BL/6 mouse. They are highly tumorigenic in immunocompetent C57BL/6 mice, and can form tumors when injected subcutaneously or orthotopically into the liver. The Hepa 1-6 cell line remains a widely used and valuable tool for studying the biology of HCC and testing potential therapies. Its high tumorigenicity and genetic and molecular similarities to human HCC make it a useful model for preclinical studies.
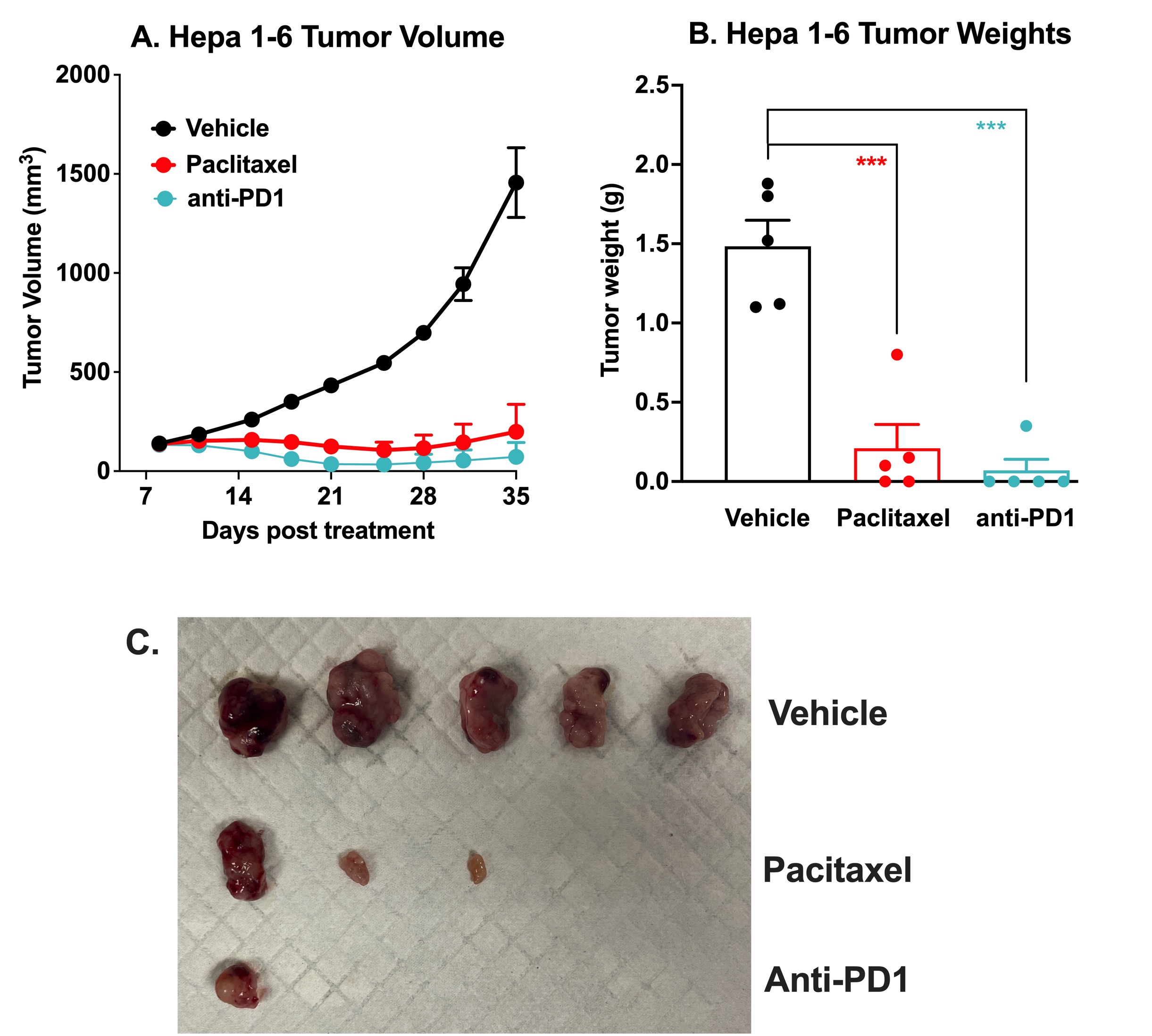
Immune Checkpoint Inhibitor and Chemotherapy Validation of the Hepa 1-6 Syngeneic Mouse Model of Liver Cancer. 10 x106 Hepa 1-6 murine hepatoma cells were subcutaneously injected into the rear flank of C57Bl/6J mice. Once the tumor size reached 100-150mm3(Day 8), mice were randomized into groups and treated with vehicle, anti-PD-1 antibody (10 mg/kg, IP once), or paclitaxel (20 mg/kg, IP twice per week). ). Tumor volume was monitored twice per week using calipers (A). At the end of the study (Day 35), animals were sacrificed, tumors excised and weighed (B,C). Data are mean ± SEM; n=5 for each group; *** p<0.001 by Student’s t-test.
Melior can initiate your Hepa 1-6 tumor model study with very short lead times and with bespoke study design to suit your needs. Including time to establish tumor-bearing mice (~X days) and typical treatment times (~Y days) these studies normally run for approximately 3-4 weeks. The Hepa 1-6 syngeneic mouse model yields highly reproducible tumor growth curves with small error bars capable of detecting small changes in growth rates will relatively small group sizes.
References:
- Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. doi:10.4103/jcar.JCar_9_16
- Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238-iv255. doi:10.1093/annonc/mdy308



 Interested in running a syngeneic liver cancer model study?
Interested in running a syngeneic liver cancer model study?