L1210 Syngeneic Leukemia Model
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Leukemia is the most common type of cancer in children and adolescents, accounting for approximately 30% of all cancers in this age group. It is characterized by an uncontrolled growth of abnormal white blood cells, which can interfere with the normal production of red blood cells, platelets, and healthy white blood cells.
Treatment options depend on the type and subtype of leukemia, as well as the patient’s age and overall health. They include chemotherapy, radiation therapy, stem cell transplant, targeted therapy, and immunotherapy. Advanced treatment options, such as CAR-T cell therapy, offer hope to patients who do not respond to standard therapies. As prevalent as these cancer types have ben in children and adolescent, leukemias also represent the cancer form that has witnessed the greatest advances over the last decade with CAR-T and other immunotherapies because of the accessibility of these tumors to the adaptive immune system. Still there remains high unmet medical needs for further advances in the available leukemia therapies. Melior syngeneic mouse leukemia model is an important tool towards this end.
The L1210 cell line is a widely used model of leukemia that was originally derived from a spontaneous leukemia in a DBA/2 mouse in 1951. This cell line has been extensively studied and has contributed significantly to our understanding of leukemia pathophysiology and the development of new treatment strategies
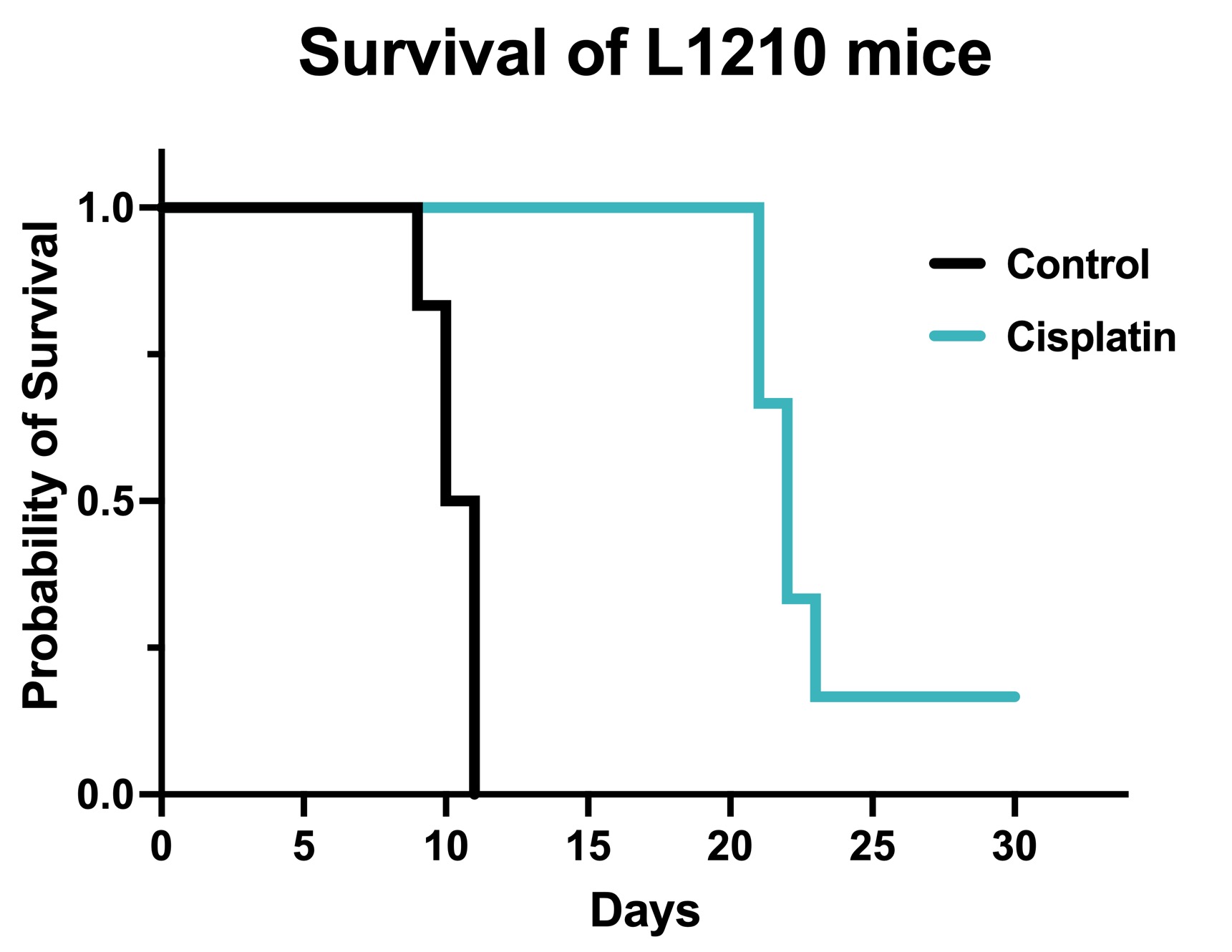
Figure 1. Chemotherapy validation in L1210 syngeneic model. 1 x106 L1210 cells were intraperitoneally injected into DBA/2 mice. The mice were randomized with body weight and the treatment was initiated 2 days after cell injection. Survival was monitored daily thereafter. Cisplatin was administered at 3 mg/kg by IP weekly for 3 weeks. Data are survival proportions from starting group size (N=6) Cisplatin significantly increased survival compared to vehicle controls. p=0.001 by Mantel-Cox Log-rank test.
As a syngeneic leukemia, Melior’s L1210 model may be ideal for evaluating CAR-T therapies other immunotherapies. Melior can initiate your L1210 leukemia model study with very short lead times and with a bespoke study design best suit your needs. Including time to establish tumor-bearing mice (1-2 weeks) and typical treatment times (3-4 weeks) these studies normally run for approximately 4-6 weeks.
References.
- American Cancer Society. (2022). What is leukemia? https://www.cancer.org/cancer/leukemia.html
- National Cancer Institute. (2022). Adult acute lymphoblastic leukemia treatment (PDQ) – health professional version. https://www.cancer.gov/types/leukemia/hp/adult-all-treatment-pdq
- American Society of Clinical Oncology. (2022). Immunotherapy for cancer. https://www.cancer.net/navigating-cancer-care/how-cancer-treated/immunotherapy-and-vaccines/immunotherapy-cancer
- Maude, S. L., Laetsch, T. W., Buechner, J., et al. (2018). Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. The New England Journal of Medicine, 378(5), 439-448
- Kantarjian, H. M., DeAngelo, D. J., Stelljes, M., et al. (2019). Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. The New England Journal of Medicine



 Interested in running an L1210 leukemia model study?
Interested in running an L1210 leukemia model study?