HCT-15 Xenograft Model
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Colorectal cancer (CRC) is a type of cancer that affects the colon and rectum, and is the third most commonly diagnosed as well as the third most lethal cancer in the world. It typically affects older adults, with the majority of cases diagnosed in people over the age of 50.
While surgery is the most common treatment for early-stage CRC, chemotherapy is often used in combination with surgery for advanced-stage CRC. It can also be used after surgery to reduce the risk of the cancer returning. Targeted therapies focus on specific molecules that are involved in the growth and spread of tumor cells. For example, anti-EGFR (epidermal growth factor receptor) therapies (cetuximab and panitumumab) work by blocking the activity of EGFR, and is often used in combination with chemotherapy for advanced-stage CRC. Anti-VEGF (vascular endothelial growth factor) therapy (bevacizumab and aflibercept) work by blocking the activity of VEGF, and is used to treat advanced-stage CRC that has spread to other parts of the body. Immune checkpoint inhibitors, which work by blocking proteins that prevent the immune system from attacking cancer cells, and which have provided revolutionary advances in some tumor types have had limited success in CRC. Ongoing research is needed to determine which patients will benefit most from these treatments. Melior’s colorectal tumor models are an important tool to serve this purpose.
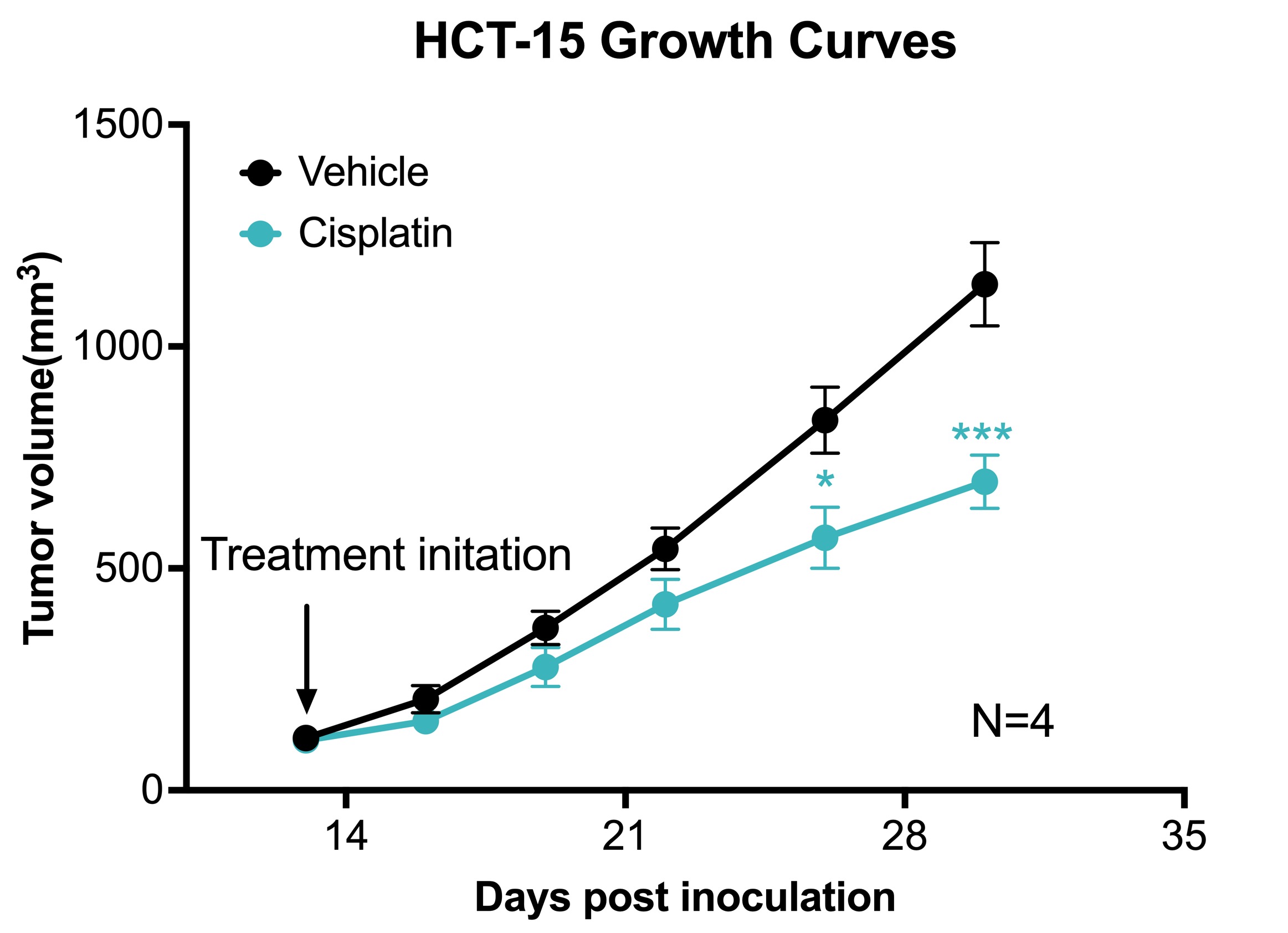
Melior has established a human colorectal tumor model using the HCT-15 cell line, to complement our syngeneic mouse colon cancer model which uses the CT26 cell line. The human HCT-15 xenograft model is conducted in nude mice (athymic; substantially lacking T cells) and is suitable for investigating cytotoxic therapeutic candidates and other non-immune modulating approaches towards tumor therapy. The HCT-15 cell line is an adenocarcinoma isolated from a male colorectal patient with a Dukes’ type C tumor.
Chemotherapy validation in human colon cancer HCT-15 xenograft model. The HCT-15 tumor model was created by subcutaneously injecting 1 x106 HCT15 into the rear flank of nude mice. Once the tumor size reached ~100mm3 (Day 13), mice were randomized into vehicle control group (treated with normal saline) and cisplatin group (3 mg/kg, IP twice/week). N=4 / group. Data area mean ± SEM. * P<0.05, *** P<0.001 by Student’s t-test.
Melior can initiate your HCT-15 tumor model study with very short lead times and with bespoke study design to suit your needs. Including time to establish tumor-bearing mice (2 weeks) and typical treatment times (2-weeks) these studies normally run for approximately 4 weeks.
References
American Cancer Society. (2021). Colorectal Cancer.
https://www.cancer.org/cancer/colon-rectal-cancer.html



 Interested in running an HCT-15 tumor model study?
Interested in running an HCT-15 tumor model study?