Patient-Derived Xenograft Models
Gain enhanced treatment insights when you leverage patient-derived xenograft (PDX) models and the patient tumor microenvironment.
Improve the clinical outcomes of your cancer therapy by preserving the genetic complexity and tissue heterogeneity of the tumor it is built to target. Make better decisions with trusted insights that ensure your cancer therapy’s early success guides authentic clinical solutions.
Validate novel cancer treatments in vivo against patient-derived cells
By directly implanting human tissue into immunocompromised mice, you preserve the tumor microenvironment, granting unparalleled access to your patient’s complex cellular network.
Better predict the clinical success of your novel cancer therapies by validating them in a PDX model that recapitulates the clinical target and ensures your most promising candidates progress and perform.
Advantages of choosing a PDX mouse model
- Test in authentic tumor conditions: The presence of stromal cells and extracellular matrix components yields conditions that closely mimic the tumor microenvironment.
- Conserve genetic architecture: PDX models maintain all the genetic and epigenetic changes in the patient’s tumor.
- Get congruent responses: Response to a given treatment is more likely to recapitulate the response of the patient’s original tumor in a clinical setting when you work with a PDX model.
Rapid results from our efficient sample-to-data pipeline
Our experts have streamlined the process of developing and validating bespoke PDX mice models that provide high-quality data.
Your patient-derived tumor sample is directly implanted into an immunocompromised or humanized mouse for tissue proliferation, eliminating the in vitro cell culture steps that promote tissue homogeneity and establishing an accurate model of your patient’s native cellular conditions. After minimal rounds of subsequent implantation and proliferation, your PDX tumor model is ready to deliver the data needed to push your therapy to the next level, getting you from a patient sample to a PDX model in approximately two to four months.
Ready to enrich your cancer R&D with custom-integrated studies?
Contact us for more information on our additional and bespoke services including IVIS imaging, PK studies, and metabolic analyses.
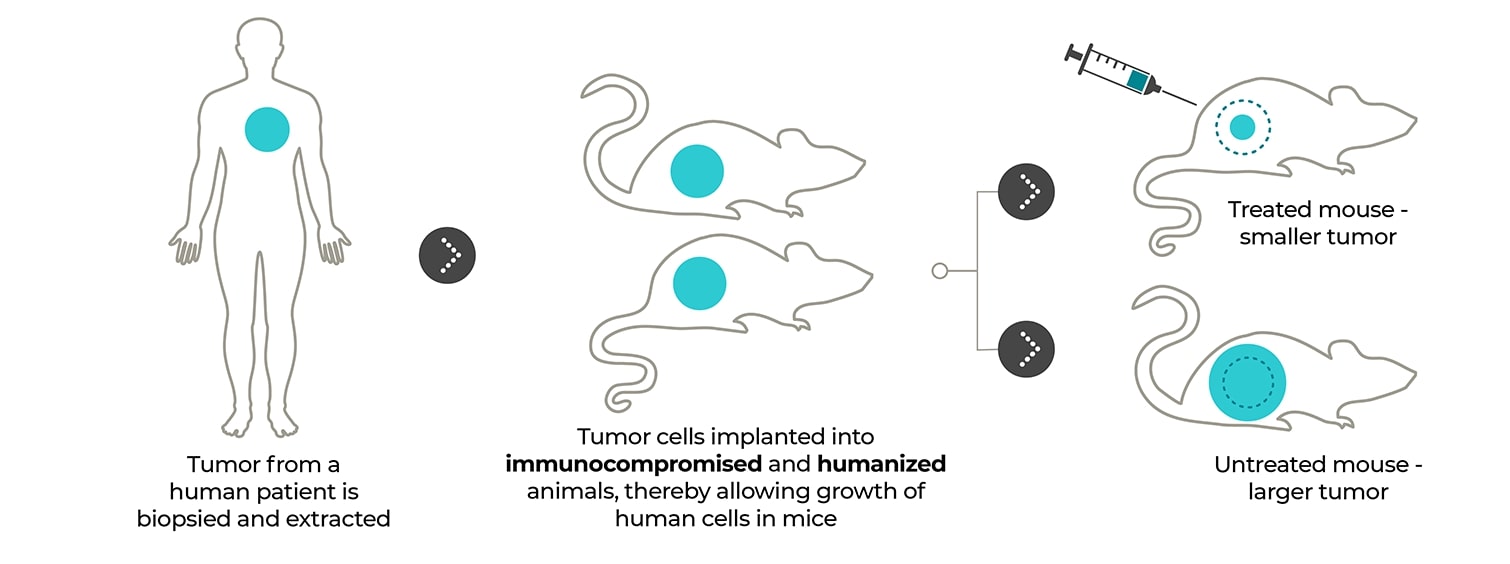
Patient-derived xenograft models: Tumors directly from the patient to the mouse. PDX models enable patient tumor tissue to be implanted directly into immunocompromised or humanized mice for access to the preserved patient tumor microenvironment and enhanced clinical insights.
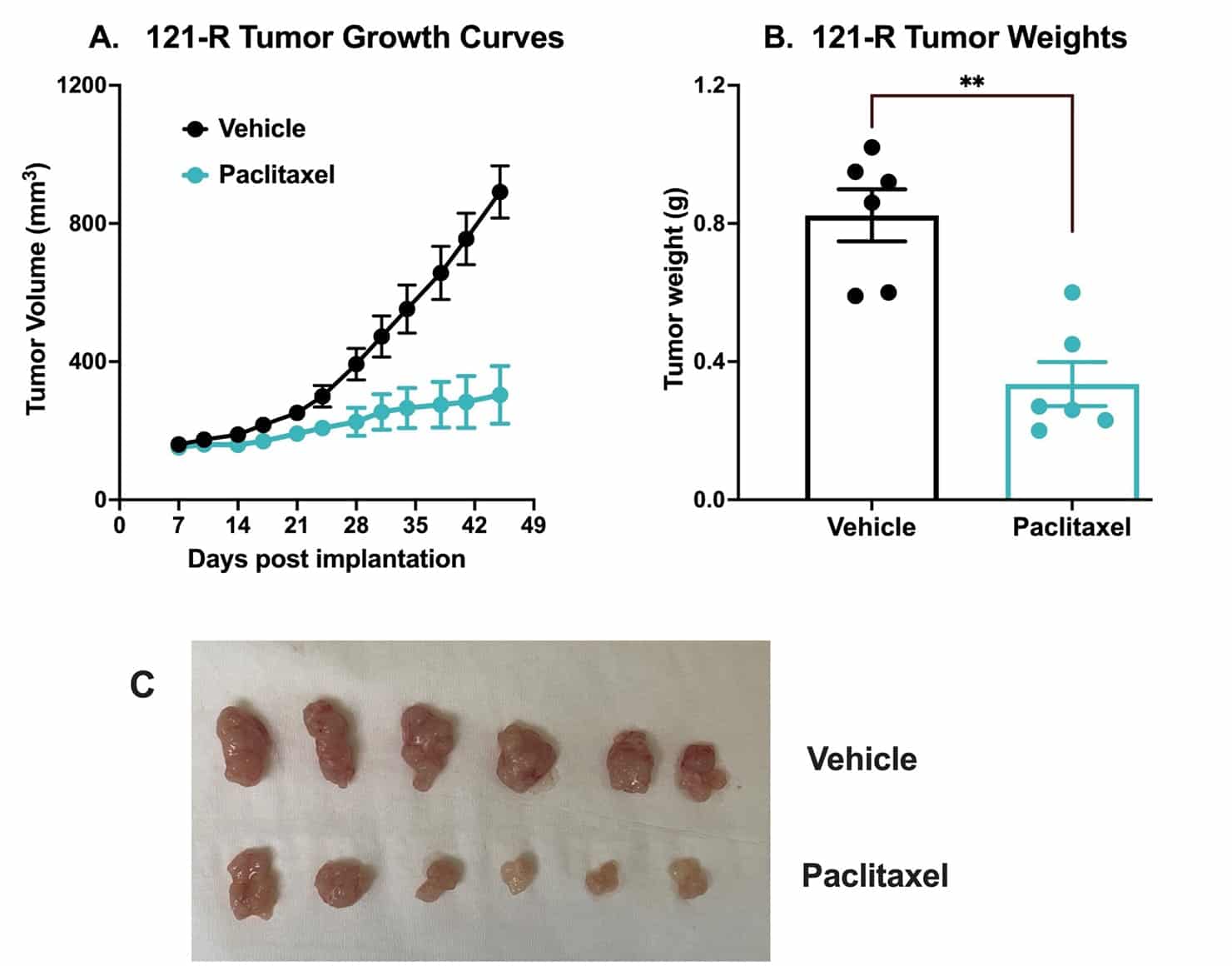
Chemotherapy validation in human colon cancer PDX 121-R subcutaneous model. This tumor was excised from a colon adenocarcinoma of a 62-year-old white male in 2017 (P0). The tumor grade /stage was designated as: pT3 pN1b Mx. The tumor tissue was subsequently passaged 3x over approximately 3 months in NSG mice (P3). For this study the mice were subcutaneously implanted with a small piece of P3 121-R tumor with Matrigel into the rear flank of NSG mice with 11G trocar.
Once the tumor size reached ~150mm3 (Day 7), the mice were randomized into vehicle control group (treated with normal saline) and paclitaxel group (20 mg/kg, IP twice/week). Tumor volume was monitored twice per week using calipers (A). At the end of the study (Day 45), animals were sacrificed, and tumors were excised and weighed (B, C). Data are mean ± SEM; n=6 /group; ** p<0.01 by Student’s t-test.
Did you know you can assess immunotherapies with PDX models?
Deciding to test your cancer therapy with a PDX mouse model doesn’t have to mean working in immunodeficient mice. We offer bespoke humanized PDX models where your patient tumor samples are implanted in mice that have been engrafted with human immune system components, enabling the study of important tumor-immune system interactions.
Frequently Asked Questions
PDX models are excellent for accurately predicting clinical success for targeted therapies such as monoclonal antibodies or combination therapies. But for a cost-effective alternative solution, cell line-derived xenograft (CDX) models have good reproducibility and are fast-growing making them ideal for high-throughput screening. You can pair your xenograft with a complementary syngeneic tumor model to quickly sample the treatment response of your most promising immunotherapies within an intact immune system. Choosing the model best suited to your R&D needs is a critical first step for validating novel cancer therapies.
PDX (patient-derived xenograft) models use real patient tissue, preserving the tumor’s complexity and offering accurate clinical insights to guide novel cancer therapy R&D. Whereas CDX (cell line-derived xenograft) models use established cancer cell lines, providing consistency and efficiency for high-throughput drug screening.
PDX mouse models offer high clinical relevance by maintaining the heterogeneity of human tumors, providing R&D scientists with access to models that closely mimic the tumor microenvironment and therefore, produce more accurate insights. Our PDX models are also offered in humanized mice, allowing immune system interaction research to be carried out early in your development pipeline.
A PDX mouse model starts with tumor tissue that has been surgically obtained from a cancer patient. This tissue is directly implanted into an immunodeficient mouse, usually subcutaneously. As the human tumor tissue grows and develops, it can be transplanted into other immunodeficient or humanized mice for further expansion and study. This process preserves the histological and genetic characteristics of the patient’s tumor, enabling accurate insights into your cancer treatment’s efficacy.





