Audiogenic Seizure in Fmr1 Knockout Mice
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Fragile X patients have a mutation in the Fmr1 gene in which the gene, (FMRP; fragile X mental retardation protein), is not expressed. FMRP is an RNA binding protein that plays a pivotal role in synaptic functioning by translational regulation of dendritic mRNAs.
Fmr1 knockout (KO) mice have an increased susceptibility to audiogenic seizures (Thomas et al., 2011; Veeraragavan et al., 2011; Osterweil et al., 2010). Moreover, Fmr1 KO mice are reported to exhibit deficits in learning and memory tests such as fear conditioning (Zhao et al., 2005; Hayashi et al., 2007; Guo et al., 2012; Gu et al., 2002). Overall, this animal model is considered to be a clinically translatable model of fragile X syndrome.
After evaluating locomotor activity, mice are placed into Audiogenic Seizure test chambers with attached alarms. After acclimating, animal behavior is recorded for a 2 minute alarm challenge, a 1 minute resting period and an additional 2 minute challenge.
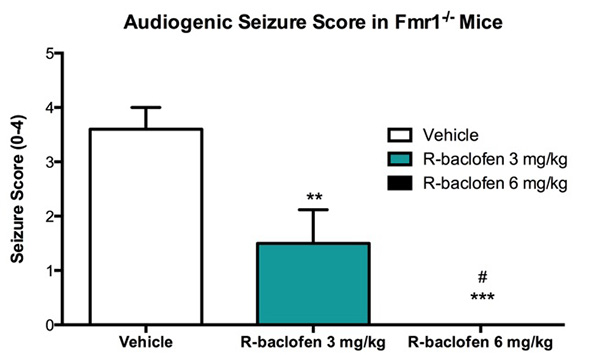
In the figure above, mice were scored based on their behavior according to a four point severity score. Animals are considered to have a seizure if the severity score is two or above. R-baclofen significantly reduces the seizure score at 3mg/kg dose and eliminates seizure at 6mg/kg dose. Data are mean ± SEM; **p<0.01, ***p<0.001 compared to vehicle; #p<0.05 R-baclofen dose response (N=15).
References
Thomas AM, Bui N, Graham D, Perkins JR, Yuva-Paylor LA, Paylor R. (2011)
Genetic reduction of group 1 metabotropic glutamate receptors alters select behaviors in a mouse model for fragile X syndrome. Behav Brain Res. 223(2):310-21
Veeraragavan S, Graham D, Bui N, Yuva-Paylor LA, Wess J, Paylor R. (2011). Genetic reduction of muscarinic M4 receptor modulates analgesic response and acoustic startle response in a mouse model of fragile X syndrome (FXS). Behav Brain Res. 228(1):1-8.
Osterweil EK, Krueger DD, Reinhold K, Bear MF. (2010). Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 30(46):15616-27.
Zhao MG, Toyoda H, Ko SW, Ding HK, Wu LJ, Zhuo M. (2005). Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci. 25(32):7385-92.
Gu Y, McIlwain KL, Weeber EJ, Yamagata T, Xu B, Antalffy BA, Reyes C, Yuva-Paylor L, Armstrong D, Zoghbi H, Sweatt JD, Paylor R, Nelson DL. (2002). Impaired conditioned fear and enhanced long-term potentiation in Fmr2 knock-out mice. J Neurosci. 22(7):2753-63.
Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. (2007). Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci U S A. 104(27):11489-94.
Guo W, Murthy AC, Zhang L, Johnson EB, Schaller EG, Allan AM, Zhao X. (2012). Inhibition of GSK3β improves hippocampus-dependent learning and rescues neurogenesis in a mouse model of fragile X syndrome. Hum Mol Genet. 2012 Feb 1;21(3):681-91.



 Interested in running the Audiogenic Seizure in Fmr1 Knockout Mice?
Interested in running the Audiogenic Seizure in Fmr1 Knockout Mice?