Fear Conditioning Test
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
The fear conditioning learning and memory model is a method for evaluating trial compounds ability to reverse a drug-induced deficit in memory.
Fear conditioning involves pairing a context and/or cue (conditioning stimuli, CS) to a shock (unconditioned stimulus, US) and later measuring the ‘fear’ response to the CS in the absence of the US. A defensive freezing or immobility response serves as a reliable measure of fear.
Contextual and auditory-cue fear conditioning depend on different neural processes, whereas contextual conditioning is hippocampally driven and cued fear conditioning is not (Kim and Fanselow, 1992; Fendt and Fanselow, 1999).
NMDA-type glutamate receptor antagonists and nicotinic cholinergic receptor antagonists (e.g MK-801) cause amnestic effects in learning and memory tests such as fear conditioning. The compounds are often used to produce deficits in these models. Several classes of drugs reverse learning and memory deficits in the Fear Conditioning Test including acetylcholinesterase inhibitors (Csernansky et al., 2005) and phosphodiesterase type 4 (PDE4; e.g. rolipram) inhibitors (Gong et al. 2004).
Contextual conditioning is considered to require a higher order form of memory involving more cognitive function than cued conditioning. In comparison, cued conditioning involves a simpler, or lower form of memory that is considered to be more reflexive in nature. In Melior’s fear conditioning model context, is established with a number of visual references in the conditioning chamber. Cuing is established with an auditory tone, paired during the training phase.
The study summarized below was designed to establish a dose of MK-801 (an NMDA receptor antagonist) that blocks contextual conditioning but not cued conditioning. Rolipram (a PDE4 inhibitor) restores the deficit created by MK-801.
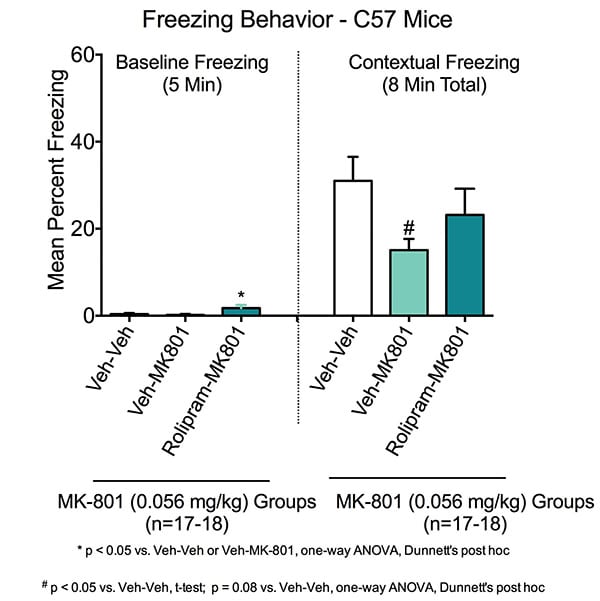
Contextual Learning in the Fear Conditioning Test. During the baseline training session on Day 1, there was minimal freezing across all groups (i.e. animals placed in chamber and received no shocks). Animals treated with rolipram had a small increase in baseline freezing.
Contextual freezing was measured on Day 2. Animals were trained in the established context of the chambers but no cued auditory tone. Animals were then placed in the chambers for 8 minutes with no shocks and freezing measured. Veh-Veh treated animals exhibited a marked increase in freezing behavior following fear conditioning training, indicating normal conditioning to the chamber context. When the entire 8-minute test period is considered, MK-801 caused impairment of contextual memory, such that Veh-MK801 animals had significantly reduced contextual freezing compared to Veh-Veh treated animals(#p<0.05) . This deficit was restored with rolipram in the Rolipram-MK801 animals.
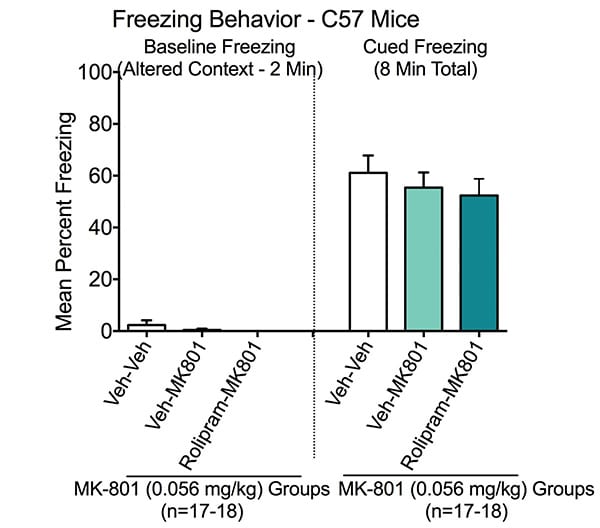
Cued Learning in the Fear Conditioning Test. Cued freezing was measured on Day 3. Baseline was first assessed in chambers in which the contextual features were modified from those used to train for contextual learning. There was minimal freezing across all groups (i.e. animals placed in modified chamber and received no shocks).
Animals were trained in the same chambers with a cued auditory tone. Veh-Veh treated animals exhibited a marked increase in freezing behavior in the presence of the auditory tone, indicating normal conditioning to the auditory cue. When the entire 8-minute test period is considered, neither MK-801 nor Rolipram imparied cued memory, which is consistent with internal validation studies.
References
Csernansky, JG, Martin, M, Shah, R, Bertchume, A, Colvin, J, Dong, H. 2005 Neuropsychopharmacology30:2135-2143.
Kim, JJ, Fanselow, MS. Modality-specific retrograde amnesia of fear. 1992 Science256:675-676.
Fendt, M, Fanselow, MS. The neuroanatomical and neurochemical basis of conditioned fear. 1999 Neurosci Biobehav Reviews23:743-760.
Gong, B, Vitolo, OV, Trinchese, F, Liu, S, Shelanski, M, Arancio, O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. 2004 J Clin Invest114:1624-1634.



 Interested in running a Fear Conditioning Test study?
Interested in running a Fear Conditioning Test study?