PLP-Induced EAE Mouse Model of Multiple Sclerosis
[/vc_row_inner]Ready to get started or looking for a custom model?
Contact us today for more information about our bespoke research models and to discuss how we can help you answer your unique research questions.
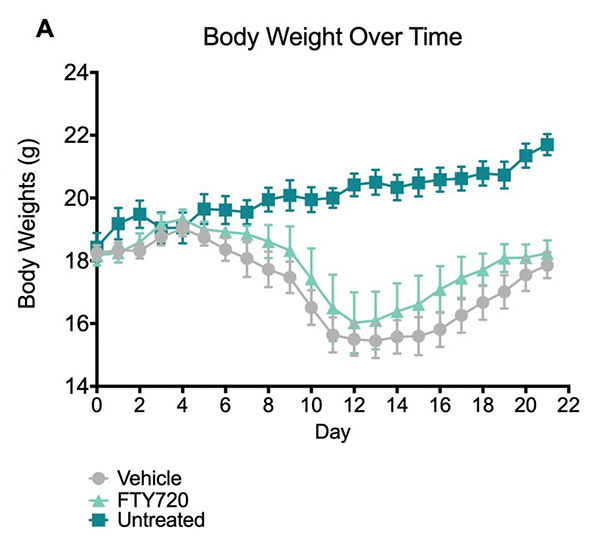
Body weights.The untreated mice showed an increase in body weight throughout the study. The FTY-720 and vehicle treated mice displayed a decrease in body weights following injection of PLP139-151/CFA, which was maintained throughout the study.
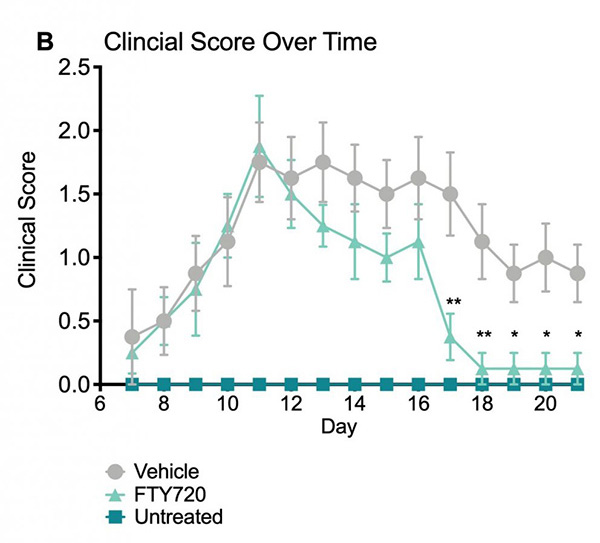
Average clinical scores over time. Clinical scores were assessed daily beginning on Day 7 and ending on Day 21. Based on the clinical score system, vehicle treated mice showed an increase in impairment severity throughout the course of the study. The FTY-720 treated mice showed a decrease in impairment following drug administration that became statistically significant when compared to vehicle treated mice on days 17-21. Data are mean ± SEM; * p<0.05, **p<0.01 compared to vehicle.
Clinical Scoring: Mice were randomized according to clinical score on Day 7 of the study. Clinical scoring was completed so that each mouse received one score per day. Clinical scores were evaluated using the following scale:
| Clinical Score | Symptoms |
|---|---|
| 0 | No clinical signs; normal activity |
| 1 | Limp tail or hind limb weakness, but not both |
| 2 | Limp tail and hind limb weakness |
| 3 | Partial hind limb paralysis |
| 4 | Complete hind limb paralysis |
| 5 | Moribund state; death by EAE |
The PLP139-151-induced EAE Multiple Sclerosis Model can be performed in mice or rats. Typical study duration is about 21 days.



 Interested in running an EAE Model of Multiple Sclerosis study?
Interested in running an EAE Model of Multiple Sclerosis study?