Formalin Analgesia Assay
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
The formalin chemical nociception assay is a commonly used analgesia assay that is typically included in a battery of analgesic tests. Formalin produces a painful irritation when injected into the skin of experimental animals that can be measured as a licking response.
Formalin nociception is typically described as occurring in two phases:
- Phase I which is an acute response to the formalin injection and occurs almost immediately after injection and lasting for between 5 to 10 minutes,
- Phase II, which lasts from approximately 20 to 40 minutes after injection is caused by a central sensitization activity.
Formalin induced chemical nociception can be attenuated by classical anti-nociceptive agents including opioids and GABA enhancers. We include the formalin assay in a battery of analgesia assays that include the thermal nociceptive assay and the touch sensitivity assay.
Ready to get started or looking for a custom model?
Contact us today for more information about our bespoke research models and to discuss how we can help you answer your unique research questions.
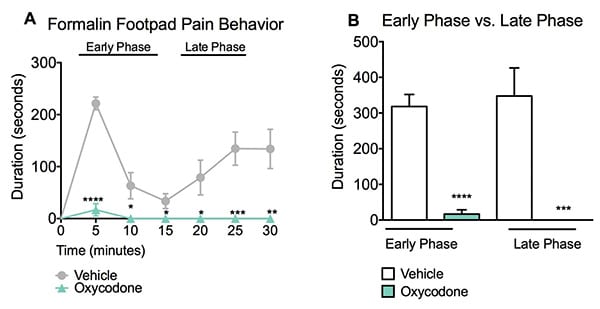
The formalin pain assay measures a compound’s analgesic effect. Vehicle treated mice peaked within the first five minutes (early phase) of monitoring with a nearly continuous display of pain behavior. Following this peak, there was a classic “no response” period that lasted for approximately 10 minutes. Vehicle treated mice then entered the late phase response period where pain behaviors were again more pronounced. Administration of Oxycodone significantly reduced the pain response in both the early and late phases. Data are mean ± SEM; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to vehicle. (N=8).
The Formalin Analgesia model is typically run in an acute mode (study completed in one day) by evaluating test articles after a single administration. The variability is relatively low and statistical significance may be achieved with group sizes of about 8 to 10 animals. It can be performed in both mice and rats.



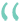 Interested in running a Formalin Analgesia study?
Interested in running a Formalin Analgesia study?