Insulin Tolerance Test (ITT)
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
The Insulin Tolerance Test (ITT) is designed to determine the whole body sensitivity of insulin receptors by measuring blood glucose levels changes before and after insulin administration. This is a standard test to determine the insulin resistance status in humans and experimental animals. This test is used to assess the insulin-sensitizing efficacy of test compounds and pharmacological agents that can modify insulin sensitivity.
Ready to get started or looking for a custom model?
Contact us today for more information about our bespoke research models and to discuss how we can help you answer your unique research questions.
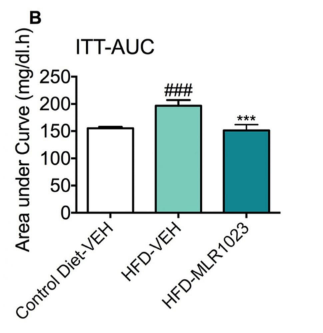
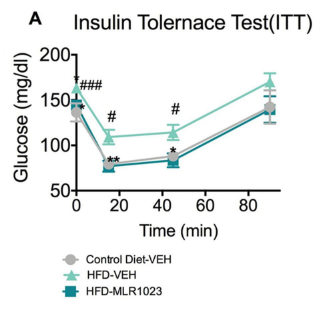
The graph above illustrates the blood glucose changes of mice with different treatments responding to an exogenous insulin challenge. All mice were fasted for 4 hours before the test. During ITT assay, mice were measured for blood glucose at 0 min (baseline), 15min, 45min and 90min post-insulin challenge. All mice were administered with an insulin solution (0.8U/kg) via S.C. injection. (A) Compared to the mice of control diet-Vehicle, mice of High-fat Diet (HFD)-Vehicle displayed a profound whole-body insulin resistance, indicating by the significantly higher blood glucose at timepoints of 0 min (baseline), 15min and 45min post-insulin injection. Compared with HFD-vehicle mice, the HFD-MLR1023 mice significantly improved insulin sensitivity, which is reflected by significantly decreased blood glucose at timepoints of -30min (baseline), 15min and 45min post-insulin injection. (B) The area under curve (AUC) were also calculated based on the glucose levels of each time point. Compared to control diet-Vehicle, the AUC value was significantly higher in HFD-Vehicle group. MLR1023 treatment markedly reduced the AUC values, which induced by HFD feeding. Data are mean±SEM and analyzed by Unpaired T-tests as applicable (*p<0.05,**p<0.01, vs. HFD-Vehicle; #p<0.05, ###p<0.001, vs. Control diet-Vehicle) (N=7-11).
MLR-1023 is a potential “next-generation” insulin sensitizer that works independently of a PPAR mechanism by activating Lyn kinase. For more information on MLR-1023, please visit our sister site, Melior Pharmaceuticals.
Frequently Asked Questions
2-4 hrs
Subcutaneous injection (SC)
Yes. The customized time points can be applied to the protocol.
Blood (<5 µL) will be acquired from a tail snip and directly applied to a glucose test strip. The blood glucose value will be read by a pre-calibrated glucometer.
Synonyms: ITT, insulin tolerance, insulin sensitivity, insulin resistance



 Interested in running an Insulin Tolerance Test study?
Interested in running an Insulin Tolerance Test study?