Oral Glucose Tolerance Test (OGTT)
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
The oral glucose tolerance test (OGTT) is a standard assay to evaluate glucose tolerability and insulin resistance. In a typical OGTT animals are first fasted and then presented with a bolus dose of glucose by oral administration. Plasma glucose levels are then monitored at several time points post-glucose challenge which forms a glucose response curve. The data can also be summarized by calculating an area-under-the-curve (AUC) for the plasma glucose. This model evaluates a test articles ability to attenuate the level of postprandial glucose excursion and thereby reduce the AUC.
Several pharmacological classes can effectively regulate this glucose tolerability response including sulfonylureas, thiazolidinediones (PPARγ agonists; glitazones), metformin (Glucophage®), GLP-1s, SGLT-2 inhibitors and DPP-4 inhibitors. These drug classes are also clinically approved for use in humans.
In the study summarized below, we illustrate the validation of a mouse OGTT model by administering a glucose challenge to mice and showing the effects of metformin.
Ready to get started or looking for a custom model?
Contact us today for more information about our bespoke research models and to discuss how we can help you answer your unique research questions.
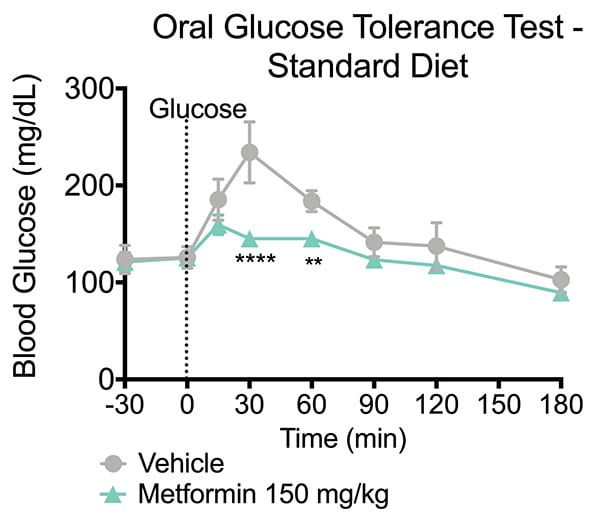
Oral Glucose Tolerance Test. Animals were fasted overnight. Metformin or vehicle was administered 15 minutes prior to glucose administration (T= -15). Glucose was administered at T=0 and at a level of 1.5g/kg. Blood glucose levels were evaluated thirty minutes prior to dosing (-30 min), immediately post-dose (0 minutes) and then 15, 30, 60, 90, 120 and 180 minutes following vehicle or Metformin administration. The Metformin treated mice exhibited a reduction in blood glucose levels after glucose challenge. Data are mean ± SEM; **p<0.01, ****p<0.0001 compared to vehicle (N=6).
Frequently Asked Questions
It can be performed in both types of mice depending on the goal of the study. Our scientists would be more than happy to help you determine which would be best for your study goals.
Absolutely! This test is non-invasive and can be done in conjunction with other tests or broader study design elements. An example of this would be in a diabetic neuropathy study in which looking at the drug’s effect on blood glucose is as important as its ability to prevent diabetic neuropathy.
Synonyms: Diabetes, Glucose



 Interested in running an Oral Glucose Tolerance Test study?
Interested in running an Oral Glucose Tolerance Test study?