Melior Discovery in vivo models of NASH
in vivo Efficacy: Animal Models
Melior is a leading contract research provider of preclinical in vivo pharmacology services using rat and mouse models of liver disease.
NASH
Non-alcoholic steatohepatitis (NASH) represents an area of high unmet medical need that quickly progressing to become the major cause of liver transplantation in the US. While the disease is defined by hepatic steatosis with focal hepatic inflammation the disease is often accompanied by INS resistance or frank diabetes, obesity and/or dyslipidemia. The hepatic steatosis progresses to liver fibrosis and ultimately liver cirrhosis.
Preclinical models of the human condition have largely been challenged by the fact that they present with only a subset of the clinical features [1]. For example, the commonly used STAM™ model, while relatively short in duration, lacks meaningful INS resistance and adiposity more characteristic of the clinical condition. Melior has established a rodent model which, to the best of our knowledge, presents with the best face validity of any animal model of NASH and, further has been validated with a clinical stage NASH candidate (OCA).
References
1. Ibrahim, S.H., Hisova, P, Malhi, H, Gores, G.J. (2016). Animal models of nonalcoholic steatohepatitis: Eat, delete, and inflame. Dig. Dis. Sci. 61(5): 1325-1336
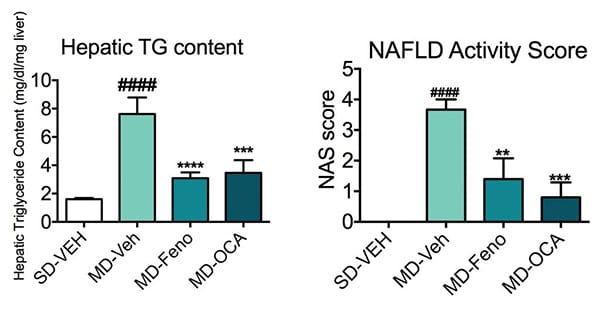
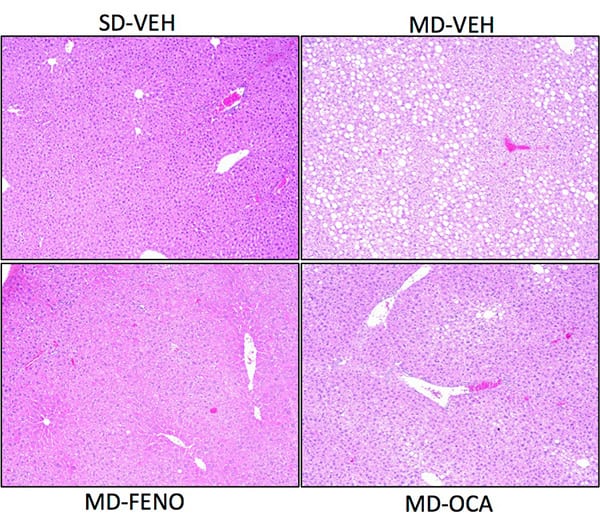
Hepatic Triglyceride (TG) and histological changes (NAS) due to the compound treatments. At the end of the study, the liver tissues were collected for hepatic TG content analysis and histology assessment for NAFLD activity score (NAS). Compared to Normal chow-fed mice, MD-fed Vehicle mice demonstrated significant higher TG deposition in liver. Compared to MD-vehicle mice, Feno (a PPAR alpha agonist) and OCA (an FXR agonist) significantly reduced the TG content in liver. Based on the pathology scores, the NAS were also calculated. Compared to Normal chow-fed mice, MD-fed Vehicle mice demonstrated significant higher NAS in liver. Compared to MD-vehicle mice, Feno and OCA significantly reduced NAS reflecting the histology improvements . Data are mean±SEM and analyzed by Unpaired T-tests as applicable (*p<0.05,**p<0.01,***p<0.001****p<0.0001, vs. MD-Vehicle; #p<0.05, ###p<0.001,#### p<0.0001, vs. SD-Vehicle).
See Additional Data on ob/ob Mouse Model of Type II diabetes
Get More Information on Melior’s NASH model and theraTRACE® Phenotypic Screening Platform: